Abstract
Purpose
This study aimed to validate the synergistic effect of ABT-737 on docetaxel using MDA-MB-231, a triple negative breast cancer (TNBC) cell line overexpressing B-cell lymphoma-2 (Bcl-2).
Methods
Western blot analysis was performed to assess expression levels of Bcl-2 family proteins and caspase-related molecules. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell cycle distribution was determined by flow cytometry analysis. Benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (z-VAD-fmk) was used for pretreatment to assess the role of caspases.
Results
Cell viability of MDA-MB-231 after combination treatment with ABT-737 and docetaxel was significantly lower than that after docetaxel or ABT-737 monotherapy based on MTT assay (both P < 0.001), with a combination index of 0.41. The proportion of sub-G1 population after combination treatment was significantly higher than that after docetaxel or ABT-737 monotherapy (P = 0.001, P = 0.003, respectively). Pretreatment with z-VAD-fmk completely restored cell viability of MDA-MB-231 from apoptotic cell death induced by combination therapy (P = 0.001). Although pro-caspase-8 or Bid did not show significant change in expression level, pro-casepase-9 showed significantly decreased expression after combination treatment. Cleaved caspase-3 showed increased expression while poly (ADP-ribose) polymerase cleavage was induced after combination treatment. However, hypoxia-inducible factor 1-alpha and aldehyde dehydrogenase 1 totally lost their expression after combination treatment.
Conclusion
Combination of ABT-737 with docetaxel elicits synergistic therapeutic effect on MDA-MB-231, a TNBC cell line overexpressing Bcl-2, mainly by activating the intrinsic pathway of apoptosis. Therefore, adjunct of ABT-737 to docetaxel might be a new therapeutic option to overcome docetaxel resistance of TNBCs overexpressing Bcl-2.
Triple negative breast cancers (TNBCs) are defined as tumors that lack expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). They consist of approximately 12% to 17% of all invasive breast cancers [1]. Chemotherapy is currently the mainstay of systemic treatment for TNBCs because endocrine therapy and HER2-targeted therapy are ineffective [2]. Patients with TNBC tend to have higher recurrence rate, shorter diseasefree survival, more rapid disease progression, and shorter overall survival [23]. Although the efficacy of conventional chemotherapy for treating TNBCs has been demonstrated, TNBCs remains a subtype with poor prognosis for which we have no known targeted agents [2]. Therefore, novel therapeutic strategies are urgently needed for TNBCs.
B-cell lymphoma-2 (Bcl-2) is the founding member of Bcl-2 family of regulator proteins that regulate cell death (apoptosis) either by inducing or inhibiting apoptotic cell death [4]. Bcl-2 was initially identified as an oncogene in human follicular B-cell lymphoma with a t(14;18) chromosome translocation [5]. Since then, its association with a variety of neoplasms including breast cancer has been reported. We have previously reported that Bcl-2 expression is positive in 68.2% of unselected breast cancer patients [6] and 39.8% of TNBC patients [7]. Although many studies have reported the prognostic role of Bcl-2 in breast cancer [89], the mechanism of action involved remains unclear.
Taxanes such as docetaxel and paclitaxel are commonly drugs used to treat TNBCs in clinical practice. Although taxanes have elicited effective initial responses for TNBCs, acquired resistance to taxanes has been a major hurdle in clinical practice [10]. ABT-737, a small molecule that mimics the action of pro-apoptotic Bcl-2 homology domain 3-only proteins, has been shown to bind and neutralize pro-survival proteins Bcl-2, Bcl-xL, and Bcl-w, but not Mcl-1 or A1 [11].
Although a few preclinical studies have recently reported that ABT-737 has promising therapeutic effect as an adjunct with taxanes for Bcl-2 positive TNBCs [121314], more validation studies are needed for its application in clinical practice. Therefore, the objective of this study was to validate the synergistic effect of ABT-737 on docetaxel using TNBC cell lines.
Six human breast cancer cell lines including MDA-MB-231, MDA-MB-468, MCF-7, BT-474, HCC-1954, and SK-BR-3 were obtained from the American Type Culture Collection (Rockville, MD, USA) and grown in standard medium supplemented with 10% heat-inactivated fetal bovine serum, 100-IU/mL penicillin, and 100-µg/mL streptomycin. Cell lines were maintained at 37℃ in a humidified atmosphere with 5% CO2. MCF-7, MDA-MB-231, and MDA-MB-468 cells were cultured in medium additionally supplemented with 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 10-µg/mL insulin. ABT-737, docetaxel, and benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (z-VAD-fmk) were purchased from Selleck Chemicals (Houston, TX, USA) and solubilized in dimethyl sulfoxide obtained from Thermo Fisher Scientific (Waltham, MA, USA). Reagent of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Harvested human breast cancer cells were washed with ice-cold phosphate-buffered saline (PBS) and total protein was extracted using radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing a protease inhibitor cocktail (Sigma-Aldrich). Concentrations of extracted proteins were measured by Bradford assay [15]. Each 40 µg of protein from cell lysate was separated by 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto nitrocellulose membrane (Thermo Fisher Scientific). Blots were blocked with 5% nonfat milk in trisbuffered saline containing 0.1% Tween 20 at room temperature for 2 hours. These membranes were subsequently incubated at 4℃ overnight with corresponding primary antibodies. Blots were then incubated with horseradish peroxidase-conjugated immunoglobulin G secondary antibody at room temperature for 1h. Following incubation with enhanced chemiluminescence solution (RPN2106, GE Healthcare, Little Chalfont, UK), protein bands were detected by ImageQuant LAS-4000 system (Fujifilm, Tokyo, Japan). Signal intensity was normalized to that of β-actin as loading control.
Cytotoxicity was assessed by MTT assay as described previously [16]. Briefly, cells were plated into a 96-well plate at density of 5 × 103 cells/well and treated with docetaxel and/or ABT-737 at indicated concentrations and time periods. A stock solution of 5-mg/mL MTT solution was prepared in PBS at pH 6.8 and kept at 4℃ in the dark. After 4-hour incubation at 37℃, a formazan solubilization buffer prepared by mixing 10% sodium dodecyl sulfate and 0.01 M HCl in PBS was added. Following an additional overnight incubation, the absorbance of each well was measured at wavelength of 570 nm in a microtiter plate reader (SpectraMax Plus 384, Molecular devices, Sunnyvale, CA, USA). Combination index (CI) was calculated by the following equation: CI = CAx/ICxA+CBx/ICxB, where CAx and CBx were the concentrations of agent A and agent B used in combination to achieve x% combinatory effect; ICxA and ICxB were concentrations for single agents to achieve the same effect. CI < 1, CI = 1, and CI > 1 indicated synergism, additive effect, and antagonism, respectively [17].
MDA-MB-231 cells were plated into 6-well plates at density of 1 × 105 cells/well and treated with docetaxel and/or ABT-737 at indicated concentrations and time periods. After washing with cold PBS, cells were harvested, fixed in 70% ethanol/PBS, and incubated at 4℃ for 30 minutes. After centrifugation at 850 g for 5 minutes at 4℃, cell pellet was incubated with 100-µg/mL RNase A (Sigma-Aldrich) and 50-µg/mL propidium iodide (Thermo Fisher Scientific) at 37℃ for 30 minutes. DNA contents of stained cells were analyzed by a flow cytometer (Cytomics FC 500, Beckman Coulter, Indianapolis, IN, USA).
All statistical analyses were conducted using IBM SPSS Statistics ver. 20.0 (IBM Inc., Armonk, NY, USA) and R software, version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). All tests were 2-sided and P-value of less than 0.05 was considered statistically significant. Graphs were created using GraphPad Prism software version 7.0 for Windows (GraphPad Software Inc., CA, USA). All experiments were performed in triplicates or more. All quantitative data are expressed as mean ± standard deviation.
MCF-7 showed only ER expression while SK-BR-3 and HCC-1954 showed only HER2 expression based on Western blot (Fig. 1A). BT-474 showed both ER and HER2 expression. MDA-MB-231 and MDA-DB-468 showed neither ER nor HER2 expression. These 2 cell lines are known to be TNBCs. MDA-MB-231 showed higher expression of Bcl-2 compared to MDA-MB-468. Although MDA-MB-231 showed weaker expression of Bcl-xL, expression levels of Bcl-w were similar. Both MDA-MB-231 and MDA-MB-468 showed biphasic responses to treatment with docetaxel. Although they were initially sensitive to the drug at concentrations up to 0.1 µM, they showed resistance to the drug at concentration of 1 µM or higher (Fig. 1B, Supplementary Table 1). At all concentrations, cell viability of MDA-MD-231 was significantly higher compared to that of MDA-MB-468 (all P < 0.05). Both cell lines showed dose-dependent sensitivities to treatment with ABT-737 (Fig. 1C, Supplementary Table 1). Cell viabilities were the same after treatment with 1 µM of ABT-737. While MDA-MB-231 showed higher (P = 0.003) cell viability after treatment with ABT-737 at concentration of 0.1 µM, it showed lower cell viability after treatment with ABT-737 at concentration between 5 and 20 µM compared to MDA-MB-468 (all P < 0.05).
Morphological changes after treatment with docetaxel and/or ABT-737 were assessed under phase contrast microscopic observation (Fig. 2A). Single treatment with docetaxel or ABT-737 barely decreased cellularity compared to control into which any solvent is added at all. Combination treatment showed prominent decrease in cellularity compared to single treatment or control. Cell viability of MDA-MB-231 after treatment was assessed by MTT assay (Fig. 2B, Supplementary Table 2). Cell viability after docetaxel or ABT-737 monotherapy was not significantly different from that of vehicle control treatment. It was not significantly different between docetaxel and ABT-737 monotherapy treatments. However, cell viability after combination treatment was significantly lower than that after docetaxel or ABT-737 monotherapy treatment (both P < 0.001). CI for docetaxel and ABT-737 was found to be 0.41.
Cell cycle distribution of MDA-MB-231 was determined by flow cytometry analysis (Fig. 3, Supplementary Table 3). The proportion of sub-G1 population after combination treatment was significantly higher than that after treatment with docetaxel (P = 0.001) or ABT-737 (P = 0.003). Combination treatment showed lower proportions of G1, S, and G2/M phases compared to docetaxel monotherapy (all P < 0.05). Although combination treatment showed lower (P = 0.001) proportion of S phase compared to ABT-737 monotherapy, the proportion of G1 phase was higher (P = 0.001) in combination treatment.
MDA-MB-231 was pretreated with z-VAD-fmk, a pancaspase inhibitor, before docetaxel and/or ABT-737 treatment. Cell viability was then measured by MTT assay (Fig. 4A, Supplementary Table 4). Monotherapy of docetaxel or ABT-737 with subtherapeutic dose barely induced cell death. Pretreatment with z-VAD-fmk showed little effect on the recovery of cell death. However, combination treatment induced significant cell death and pretreatment with z-VAD-fmk completely restored MDA-MB-231 from apoptotic cell death induced by combination therapy (P = 0.001). Cell cycle distribution was also analyzed by flow cytometry analysis to investigate the role of z-VAD-fmk in apoptotic cell death using MDA-MB-231 (Figs. 4B, 4C, Supplementary Table 5). Although the decrease of sub-G1 population by pretreatment with z-VAD-fmk was minimal after docetaxel treatment, sub-G1 population was reduced prominently by pretreatment with z-VAD-fmk after ABT-737 treatment. Combination treatment also prominently decreased sub-G1 population after pretreatment with z-VAD-fmk.
Western blot analysis was performed to validate changes in expression of caspase-related molecules (Fig. 5). Expression levels of pro-caspase-8 and Bid (a substrate of caspase-8) were decreased minimally after combination treatment for 48 hours. Pro-casepase-9 showed decreased expression at 24 hours after combination treatment. Its decrease was more prominent at 48 hours after combination treatment. Cleaved caspase-3, an active form of caspase-3, showed increased expression at 24 hours and 48 hours after combination treatment. Its expression was more prominent after ABT-737 monotherapy compared to docetaxel monotherapy at 24 hours and 48 hours. Cleaved caspase-3 induces poly (ADP-ribose) polymerase (PARP) cleavage. Accordingly, PARP showed both 116 kDa and 89 kDa bands at 24 h and 48 h after combination treatment. Notably, hypoxia-inducible factor 1-alpha (HIF-1α) and aldehyde dehydrogenase 1 (ALDH1) totally lost their expression at 48 hours after combination treatment.
Achieving enhanced treatment outcome for TNBCs is one of major unmet clinical needs in breast cancer. TNBC is a diagnosis of exclusion based on lack of ER, PR, and HER2. It consists of heterogeneous subgroups [1819]. TNBCs could be classified as Bcl-2 positive or Bcl-2 negative TNBCs based on immunohistochemical analysis for the expression of Bcl-2. Previously, we have reported that almost 40% of TNBCs show positive expression of Bcl-2 [7]. Therefore, Bcl-2 could be a potential therapeutic target in Bcl-2 positive TNBCs. This study revealed that ABT-737, an anti-Bcl-2 drug, showed synergistic effect with docetaxel in MDA-MB-231 TNBC cells overexpressing Bcl-2. This finding suggests that Bcl-2 might be a therapeutic target to overcome taxane resistance in Bcl-2 positive TNBCs. In this study, MDA-MB-231 and MDA-MB-468 were TNBC cell lines. The expression of Bcl-2 was more prominent in MDA-MB-231 compared to that in MDA-MB-468. As MDA-MB-231 was more resistant to docetaxel but more sensitive to ABT-737 compared to MDA-MB-468, MDA-MB-231 was chosen for this study to reveal the synergistic effect between docetaxel and ABT-737. A previous study has reported that acquired resistance to paclitaxel is mediated by altered expression of Bcl-2 protein family members either by increased levels of anti-apoptotic Bcl-2 proteins such as Bcl-2 and Bcl-xL or by decreased levels of pro-apoptotic Bim in breast cancer cells [14]. Another study has reported that Bcl-2 is a better target of ABT-737 compared to Bcl-xL or Bcl-w, implying different apoptosis sensitivity of Bcl-2 family proteins to ABT-737 in leukemia cell lines [20].
Although subtherapeutic doses of ABT-737 and docetaxel had little treatment effect on MDA-MB-231 cells, combination therapy of these 2 drugs significantly decreased cell viability. The synergistic effect between ABT-737 and taxanes has been occasionally reported. A previous study has shown that ABT-737 treatment combined with docetaxel can decrease tumor growth and increase survival of mice bearing Bcl-2-expressing human breast tumor xenografts [12]. Bcl-xL, another target of ABT-737, has been reported to be expressed in both responsive and nonresponsive tumors. In addition, administration of ABT-263, an orally available analog of ABT-737, following shortterm paclitaxel treatment has been found to be an effective therapeutic strategy for TNBCs using MDA-MB-231 cells [13]. Another study has reported that ABT-737 can engage mitochondrial apoptosis pathway to restore sensitivity to paclitaxel in breast cancer cell lines with acquired paclitaxel resistance [14]. The mechanism by which ABT-737 synergizes with docetaxel to induce enhanced therapeutic effect is not established yet. Destabilization of interactions among Bim, Bcl-2, and Mcl-1 might be involved. Combination therapy of ABT-737 with docetaxel which competitively binds hydrophobic groove of Bcl-2, Bcl-xL, and Bcl-w, but not Mcl-1 or A1, can lead to release of Bim from Bcl-2 [12]. In this study, the CI of ABT-737 and docetaxel was found to be 0.41, indicating synergistic effect between these two drugs.
In the present study, cell cycle analysis showed that the synergistic effect of the combination therapy was mainly by apoptotic cell death represented by increased sub-G1 proportion. An intact death pathway is required for successful embryonic development and maintenance of normal tissue homeostasis. Main death pathways encompass apoptosis, necrosis, and autophagy [2122]. A previous study has reported that paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells characterized by a complete absence of caspase-mediated apoptotic cell death in paclitaxel-resistant MCF-7 cells [23]. The characteristic phenotype of apoptotic cells includes nuclear condensation and pyknosis followed by DNA fragmentation [24]. Assays that can detect DNA fragmentation are techniques that are suitable for apoptosis detection [24]. In this study, the proportion of sub-G1 was significantly increased in combination therapy compared to docetaxel monotherapy. This finding suggests that ABT-737 could sensitize MDA-MD-231 cells to docetaxel treatment by increasing apoptotic cell death.
Combination therapy of ABT-737 and docetaxel induced caspase-dependent cell death which was totally restored by z-VAD-fmk, a pan-caspase inhibitor. This finding suggested that the synergistic effect of combination treatment was mainly by apoptotic cell death rather than other types of cell death such as necrosis or autophagy. Activation of caspases is one of prominent biochemical events linked to apoptotic cell death, mitochondrial membrane permeabilization, internucleosomal DNA fragmentation, and surface exposure of phospholipid phosphatidylserine [24].
Apoptosis is a major type of programmed cell death. It can be activated via 2 different pathways: extrinsic and intrinsic. The extrinsic (also called death receptor) pathway is initiated via a death receptor family member. Caspase-8 is the major caspase involved in this pathway. The intrinsic (also called mitochondrial) pathway is engaged by a wide array of stimuli, including cytokine deprivation, DNA damage, and endoplasmic reticulum stress. Caspase-9 is the major caspase involved in this pathway [2125]. Activation of caspase-3 is essential for the initiation of apoptotic cell death. It is a common downstream molecule of both caspase-8 and caspase-9. This study showed that combination therapy of ABT-737 with docetaxel induced activation of intrinsic pathway of apoptosis without affecting the extrinsic pathway. MDA-MB-231 treated with combination therapy of ABT-737 and docetaxel showed decreased expression of pro-caspase-9 but increased expression of cleaved caspase-3 without significant changes in the expression of pro-caspase-8 or Bid. Activated caspase-3 also induced PARP cleavage. A previous study has reported that caspase-3 apoptotic foci in tumors are increased in mice bearing TNBC xenografts overexpressing Bcl-2 after treatment with combination therapy of ABT-737 and docetaxel [12]. Another paper has reported that paclitaxel plus ABT-737 treatment not only activates caspase-3 and caspase-9, but also activates caspase-8 in MDA-MB-468 TNBC cell line [14]. In apoptotic cascades, caspase-3 is one of the main enzymes cleaving PARP, leading to apoptotic cell death [26].
This study showed that HIF-1α and ALDH1 were totally suppressed by combination treatment of ABT-737 and docetaxel. Hypoxia-inducible factors (HIFs) consist of a highly regulated HIF-1α or HIF-2α subunit which can form heterodimer with constitutively expressed HIF-1β subunit. HIF-1α is known to be associated with chemotherapy resistance [27]. A previous study has reported that paclitaxel treatment induces upregulation of HIF in MDA-MB-231 cells, leading to increased expression of multidrug resistance 1 and chemotherapy resistance [27]. That study also showed that coadministration of HIF inhibitors could overcome the resistance of breast cancer stem cells to paclitaxel both in vitro and in vivo, leading to tumor eradication. Aldehyde dehydrogenase is a gene superfamily of phase I oxidizing enzymes responsible for detoxification of biogenic and xenogenic aldehydes [28]. ALDH1 has been identified as a breast cancer stem cell marker as well as a predictor of poor clinical outcome [29]. Previous studies have reported that ALDH1 positive breast cancer patients show significant higher resistance to neoadjuvant chemotherapy [30]. Downregulation of HIF-1α and ALDH1 could play an important role in the synergistic effect between ABT-737 and docetaxel in the combination therapy on TNBC cells. Further studies are needed to unveil the plausible mechanisms of action.
In conclusion, ABT-737, an anti-Bcl-2 drug, could ameliorate docetaxel resistance of MDA-MB-231, a TNBC cell line overexpressing Bcl-2. Combination therapy of ABT-737 with docetaxel could elicit synergistic therapeutic effect mainly by activating the intrinsic pathway of apoptotic cell death. Therefore, adjunct of ABT-737 to conventional taxane chemotherapy agents might be used as a new therapeutic option for TNBCs with high expression levels of Bcl-2. Further studies are needed to validate these results.
Figures and Tables
 | Fig. 1Western blot analysis for ER, HER2, Bcl-2, Bcl-xL, and Bcl-w using 6 breast cancer cell lines and MTT assay using MDA-MB-231 and MDA-MB-468 after docetaxel or ABT-737 monotherapy. (A) After lysing harvested cells (1 × 106 cells per each cell line), equal amount of cell lysate extracted from 6 breast cancer cell lines using radioimmunoprecipitation assay buffer was subjected to Western blot analysis using specific antibodies. Signal intensity was normalized to that of β-actin as loading control. MTT assay was performed using MDA-MB-231 and MDA-MB-468 (5 × 103 cells per each cell line) after treatment with indicated concentration of docetaxel (B) and ABT-737 (C) for 72 hours. Cell viability (%) was expressed as a percentage of viable cell proportion for treated sample compared to that of untreated control. Values correspond to mean ± standard deviation of three independent experiments. Black squares indicate MDA-MB-231. White squares indicate MDA-MB-231. Statistical significance was assessed by unpaired Student t-test. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; Bcl-2, B-cell lymphoma-2; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NS, not significant. *P < 0.05. **P < 0.01. ****P < 0.0001. |
 | Fig. 2Cytotoxicity evaluation of docetaxel, ABT-737, and combination treatment using MDA-MB-231. (A) MDA-MB-231 cells (5 × 103) were treated with 10 nM docetaxel and/or 1 µM ABT-737 for 48 hours. Cellular morphology was observed by phase contrast microscopy (×100). White arrow indicates a suspended shrunk cell. Black arrow points to an attached surviving cell. (B) MDA-MB-231 cells (5 × 103) were treated with 20 nM docetaxel and/or 1 µM ABT-737 for 72 hours. Cell viability was assessed by MTT assay. Absorbance was measured at wavelength of 570 nm. Values correspond to mean ± standard deviation of three independent experiments. Statistical significance was assessed by unpaired Student t-test. NS, not significant. ***P < 0.001. |
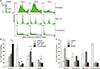 | Fig. 3Cell cycle analysis. (A) MDA-MB-231 cells (1 × 105) were treated with 10 nM docetaxel and/or 1 µM ABT-737 for indicated time periods and stained with 50 µg/mL of propidium iodide including 100 µg/mL of RNase A for 30 minutes at 37℃. Top, middle, and bottom rows were treated with ABT-737, docetaxel, and both drugs, respectively. Graphs depict data of flow cytometry results after treatment for 72 hours according to cell cycle (B) and treatment agents (C). Values correspond to mean ± standard deviation of 3 independent experiments. Statistical significance was assessed by unpaired Student t-test. NS, not significant. *P < 0.05. **P < 0.01. |
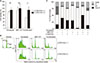 | Fig. 4MTT assay and cell cycle analysis after docetaxel, ABT-737, and combination treatment using MDA-MB-231 with or without pretreatment of z-VAD-fmk. Twenty µM of z-VAD-fmk was pretreated for 1 hour before 10 nM docetaxel, 1 µM ABT-737, or combination treatment for 48 hours. (A) Cell viability after treatment was determined by MTT assay. Statistical significance was assessed by unpaired Student t-test. Values correspond to mean ± standard deviation of five independent experiments. (B) After treatment with docetaxel, ABT-737, or combination therapy, MDA-MB-231 cells (1 × 105) were stained with 50 µg/mL of propidium iodide with (bottom) or without (top) pretreatment of 20 µM z-VAD-fmk. (C) Percentage of each phase is shown as 100% stacked bar chart. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NS, not significant. **P < 0.01. |
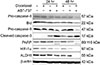 | Fig. 5Expression of apoptosis related molecules by Western blot. MDA-MB-231 cells were treated with 10 nM docetaxel, 1 µM ABT-737, or combination of the two drugs for 24 hours and 48 hours. After lysing harvested cells (1 × 106), 40 µg of protein from each cell lysate was loaded and blotted with specific antibodies, including caspase-8, caspase-9, cleaved caspase-3, Bid, and PARP antibodies. Expression levels of ALDH1 and HIF-1α were also tested under the same condition. β-actin was used as loading control. PARP, poly (ADP-ribose) polymerase; ALDH1, aldehyde dehydrogenase 1; HIF, hypoxia-inducible factor. |
ACKNOWLEDGEMENTS
This work was supported by a multidisciplinary research grant-in-aid from the Seoul Metropolitan Government - Seoul National University Boramae Medical Center (02-2016-8).
We appreciate valuable discussion from the members of the Boramae hospital Breast cancer Study group (BBS).
All BBS members belong to Seoul Metropolitan Government - Seoul National University Boramae Medical Center.
Their respective departments are as follows:
Ki-Tae Hwang (Department of Surgery), Bo Kyung Koo (Department of Internal Medicine), Young A Kim (Department of Pathology), Jongjin Kim (Department of Surgery), Eun Youn Roh (Department of Laboratory Medicine), Sung Bae Park (Department of Neurosurgery), Jin Hyun Park (Department of Internal Medicine), Han Mo Sung (Department of Surgery), Bumjo Oh (Department of Family Medicine), So Won Oh (Department of Nuclear Medicine), Sohee Oh (Department of Biostatistics), Jong Yoon Lee (Department of Radiology), Ji Hyun Chang (Department of Radiation Oncology), Se Hee Jung (Department of Rehabilitation Medicine), Young Jun Chai (Department of Surgery), In Sil Choi (Department of Internal Medicine), A Jung Chu (Department of Radiology), Kyu Ri Hwang (Department of Obstetrics and Gynecology), Eunjoo Hwang (Department of Surgery), Seong-Hye Hwang (Department of Surgery).
References
1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010; 363:1938–1948.

2. Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010; 7:683–692.

3. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434.

4. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014; 15:49–63.

5. Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984; 226:1097–1099.

6. Hwang KT, Woo JW, Shin HC, Kim HS, Ahn SK, Moon HG, et al. Prognostic influence of BCL2 expression in breast cancer. Int J Cancer. 2012; 131:E1109–E1119.

7. Hwang KT, Han W, Kim J, Moon HG, Oh S, Song YS, et al. Prognostic influence of Bcl2 on molecular subtypes of breast cancer. J Breast Cancer. 2017; 20:54–64.

8. Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010; 103:668–675.

9. Callagy GM, Webber MJ, Pharoah PD, Caldas C. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008; 8:153.

10. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281.

11. Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009; 9:321–326.

12. Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012; 109:2766–2771.

13. Panayotopoulou EG, Muller AK, Borries M, Busch H, Hu G, Lev S. Targeting of apoptotic pathways by SMAC or BH3 mimetics distinctly sensitizes paclitaxel-resistant triple negative breast cancer cells. Oncotarget. 2017; 8:45088–45104.

14. Kutuk O, Letai A. Alteration of the mitochondrial apoptotic pathway is key to acquired paclitaxel resistance and can be reversed by ABT-737. Cancer Res. 2008; 68:7985–7994.

15. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254.

16. Witham J, Valenti MR, De-Haven-Brandon AK, Vidot S, Eccles SA, Kaye SB, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007; 13:7191–7198.

17. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984; 22:27–55.

18. Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015; 121:8–16.

19. Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clin Cancer Res. 2014; 20:782–790.

20. Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis. 2012; 3:e366.

22. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004; 116:205–219.
23. Ajabnoor GM, Crook T, Coley HM. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012; 3:e260.

24. Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011; 10:221–237.

25. Ichim G, Tait SW. A fate worse than death: apoptosis as an oncogenic process. Nat Rev Cancer. 2016; 16:539–548.

26. Herceg Z, Wang ZQ. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res. 2001; 477:97–110.
27. Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014; 111:E5429–E5438.

28. Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014; 94:1–34.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1–5 can be found via https://www.astr.or.kr/src/sm/astr-95-240-s001.pdf.
Supplementary Table 1
Cell viability (%) of MDA-MB-231 and MDA-MB-468 after treatment with docetaxel and ABT-737 by MTT assay (Fig. 1B, C)
Supplementary Table 2
MTT absorbance at the wavelength of 570 nm after treatment with docetaxel, ABT-737, and combination using MDA-MB-231 (Fig. 2B)
Supplementary Table 3
Cell cycle analysis (% of each phase) after treatment with docetaxel, ABT-737, and combination using MDA-MB-231 cells (Fig. 3B, C)
Supplementary Table 4
Cell viability (%) after treatment with docetaxel, ABT-737, and combination using MDA-MB-231 with or without pretreatment of z-VAD-fmk by MTT assay (Fig. 4A)
Supplementary Table 5
Cell cycle analysis after treatment with docetaxel, ABT-737, and combination using MDA-MB-231 with or without pretreatment of z-VAD-fmk (Fig. 4C)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download