Abstract
Objective
To compare the therapeutic efficacy between conventional transarterial chemoembolization (cTACE) and combined therapy using cTACE and radiofrequency ablation (RFA) in ultrasound (US)-invisible early stage hepatocellular carcinoma (HCC).
Materials and Methods
From January 2008 to June 2016, 167 patients with US-invisible early stage HCCs were treated with cTACE alone (cTACE group; n = 85) or cTACE followed by immediate fluoroscopy-guided RFA targeting intratumoral iodized oil retention (combined group; n = 82). Procedure-related complications, local tumor progression (LTP), time to progression (TTP), and overall survival (OS) were compared between the two groups. Multivariate analyses were performed to identify prognostic factors.
Results
There was no major complication in either group. The cTACE group showed higher 1-, 3-, and 5-year LTP rates than the combined group; i.e., 12.5%, 31.7%, and 37.0%, respectively, in the cTACE group; compared to 7.3%, 16.5%, and 16.5%, respectively, in the combined group; p = 0.013. The median TTP was 18 months in the cTACE group and 24 months in the combined group (p = 0.037). Cumulative 1-, 3-, and 5-year OS rates were 100%, 93.2%, and 87.7%, respectively, in the cTACE group and 100%, 96.6%, and 87.4%, respectively, in the combined group (p = 0.686). Tumor diameter > 20 mm and cTACE monotherapy were independent risk factors for LTP and TTP.
Ultrasound (US) is the most frequently used guidance modality for radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC). However, US-guided RFA is not technically feasible in many patients with early-stage HCCs because of unfavorable tumor location and/or poor conspicuity (12). Computed tomography (CT) and fusion imaging can be alternative guiding tools for percutaneous RFA, but they still have their own limitations, which preclude their application in some proportion of patients (345). In tumors where RFA is not feasible due to the lack of adequate guiding tools, conventional transarterial chemoembolization (cTACE) has been performed as a salvage treatment for tumor control and survival prolongation even though cTACE is generally considered as a palliative treatment option (6).
Recently, ablation therapy combined with cTACE is gaining acceptance as an effective treatment for HCC (7891011). cTACE decreases blood flow to tumors, which reduces perfusion-mediated tissue cooling and permits the achievement of a larger ablation area by subsequent ablation (12). This strategy was initially developed for the treatment of intermediate and large tumors (> 3 cm) in which complete tumor eradication by RFA alone could be challenging (12). Thus, in general, although combined therapy is not recommended for small HCCs (13), it can provide a technical benefit in treating small HCCs infeasible for US-guided RFA. Intra-tumoral retention of iodized oil after cTACE provides radiographic contrast to the index lesion, and thus, can serve as a landmark to facilitate targeting an index tumor under fluoroscopic or CT guidance. Recently, several studies demonstrated that this strategy can convert RFA-infeasible tumors into RFA-feasible tumors in most cases (214151617). However, these studies focused on only the technical feasibility of this method and did not demonstrate clinical benefit over cTACE monotherapy. Moreover, a recent study showed that cTACE monotherapy and RFA can provide comparable patients' survival benefit in early HCC (18). Thus, the benefits of “additional RFA” when RFA becomes feasible after cTACE are still unclear. In this study, therefore, we compared the therapeutic efficacy between combined therapy and cTACE monotherapy for US-invisible early-stage HCC.
This retrospective study was approved by the Seoul National University Bundang Hospital Institutional Review Board. The requirement to obtain informed consent was waived. Our hospital database search yielded 4090 cTACE monotherapies and 430 combined therapies between January 2008 and June 2016. We selected 187 patients based on the following inclusion criteria: 1) treatment-native HCC of Barcelona Clinic Liver Cancer Stage 0 or A (BCLC) stage 0 or A; 2) US-invisible tumor; and 3) patients who underwent cTACE monotherapy or combined therapy as a first-line treatment. Twenty patients were excluded for the following reasons: 1) follow-up less than 6 months after the procedure (n = 12) or 2) existence of other primary malignancy (n = 8). Finally, 167 patients with 201 HCCs were enrolled in this study. The diagnosis of HCC was based on the guidelines of the American Association for the Study of Liver Diseases as follows: typical vascular pattern (hypervascularity in the arterial phase and washout in the portal/delayed phase) of liver nodule in at least one dynamic CT or MRI (19). All tumors were invisible on US because of their unfavorable location (n = 146; subphrenic location [n = 97], subcapsular location [n = 49] or iso-echogenicity [n = 55]).
In our institution, the treatment decision for HCC patients is made by a tumor board consisting of hepatologists, hepatobiliary surgeons, and radiologists. RFA is considered only when the patients are not eligible for surgical treatment. All candidates for RFA underwent a planning US examination to confirm the feasibility of the procedure 1–2 days in advance. In cases where the index tumor was not visualized on the planning US, information about cTACE and combined therapy including its potential benefits and adverse effects was given to the patients. The final decision for treatment was made 1 day before the procedure depending on the patients' preferences. As a result, 85 patients with 105 tumors underwent cTACE monotherapy (cTACE group) and 82 patients with 96 tumors underwent combined therapy (combined group) as a first-line treatment.
Characteristics of enrolled patients and their tumors are summarized in Table 1. The two groups were not significantly different in terms of etiology of liver disease, liver function, serum value of tumor marker, or number and size of the tumors.
All procedures were performed on an inpatient basis by two interventional radiologists with 15 and 10 years' experience in TACE and RFA at the beginning of this study. Written informed consents were obtained from patients and/or their family members before the procedures.
A femoral arterial access was obtained with a 5-Fr vascular sheath. Following this, a digital subtraction angiography examination was performed after catheterization of the celiac and superior mesenteric arteries with a 5-Fr catheter (RH or Cobra, Cook Medical, Bloomington, IN, USA). A 2.0 Fr (Progreat, Terumo, Tokyo, Japan) or a 3.0 Fr (Renegade, Boston Scientific, Marlborough, MA, USA) microcatheter was then used to select the segmental or subsegmental tumor-feeding arteries. Emulsions of iodized oil (2–5 cc; Lipiodol Ultra Fluid, Andre Guerbet, Aulnay-sous-Bois, France) and doxorubicin hydrochloride (10–30 mg; Adriamycin, Dong-A Pharm, Seoul, Korea) were infused. The amount of emulsion was decided based on tumor size and vascularity. The tumor-feeding artery was then embolized with gelatin sponge particles (Cali-Gel, Alicon, Zhejiang, China) until arterial flow stasis was achieved. Completion angiography was performed to make sure all tumor-feeding arteries were embolized.
In the combined group, cTACE was performed in same manner as was done for the cTACE group followed immediately by RFA in the same session. The patients received intravenous remifentanil and midazolam, which were administered incrementally from 0.05 µg/kg/min to 0.1 µg/kg/min and 1–5 mg, respectively, until adequate conscious sedation was achieved per the operator's discretion. Pre- or post-procedural prophylactic antibiotics were not routinely administered.
Radiofrequency ablation was performed under fluoroscopic guidance with a flat-panel monoplane angiographic suit (Allura Xper, Philips Healthcare, Best, The Netherlands). We used a 17-gauge internally-cooled electrode with a manually adjustable active tip of 0.5–3 cm (n = 56; Viva, Starmed, Goyang, Korea) or an expandable 10-hooks LeVeen needle (n = 40; Boston Scientific). Since fluoroscopy provides only two-dimensional information, multiple projections were required for three-dimensional targeting. After an appropriate RF electrode entry site was marked on the patient's skin under US guidance (Logiq E9, GE Healthcare, Milwaukee, WI, USA), the RF electrode was advanced a few centimeters into the liver parenchyma aiming at the iodized oil accumulated in the index tumor under anteroposterior projection of fluoroscopy. On lateral and oblique projection, the anteroposterior direction of the electrode was adjusted. The electrode direction was adjusted repeatedly via obtaining multiple fluoroscopic projections for the RF electrode to reach the index tumor. For subphrenic tumors, an oblique approach from the lower intercostal space was used rather than a transthoracic approach. Maneuvers were done under US guidance to avoid the traversal of critical structures, such as large vessels and the gallbladder. After confirming an adequate position of the electrode, RF energy was applied for 8–12 minutes for each tumor (Fig. 1). At the end of the procedure, the electrode tract was cauterized to prevent bleeding and tumor seeding.
The patients in the combined group were examined using contrast-enhanced CT on the day after the procedure to evaluate immediate therapeutic responses and procedure-related complications. In both groups, follow-up contrast-enhanced CT or MRI was performed 1 month after the procedures, and then at 2–3 month intervals. Laboratory tests including liver function tests and serum alpha-fetoprotein were obtained every 2–3 months. In cases of recurrence, patients were offered other treatment options to choose from such as TACE, RFA, combined therapy, surgical resection, liver transplantation, or radiation therapy depending on their underlying liver function and recurrent tumor features.
Technical success of cTACE was defined as the successful selection of segmental or subsegmental tumor-feeding artery and administration of chemoembolic agents as planned (20). Technical success of combined therapy was defined as accurate RF electrode placement at the index tumor with iodized oil retention and ablation completed under fluoroscopic guidance. Technique efficacy of combined therapy was defined as eradication of tumor enhancement with a surrounding hypo-attenuating non-enhancing area on a follow-up CT obtained one day later (21). One-month tumor response was evaluated and classified according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) for HCC in both groups (22). Local tumor progression (LTP) was defined as any enhancing viable tumor located in or adjacent to the treated area where complete uptake of iodized oil or ablation zone was noted on follow-up CT or MRI. Intrahepatic distant recurrence was defined as development of a new tumor in the liver separate from the treated tumor and distant metastasis was defined as new extrahepatic tumor. Time to progression (TTP) was defined as the time elapsed between initial treatment and LTP, intrahepatic distant recurrence, or distant metastasis. Overall survival (OS) was defined as the time from either cTACE or combined therapy to death, and patients alive at the end of follow-up were censored. All patients were followed up for more than 6 months after the procedures.
Liver function test results including aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin (TB), prothrombin time (PT) before and 1 month after the procedure were compared to evaluate changes in liver function after the procedure. Complications were assessed according to the guidelines of the Society of Interventional Radiology (21). A major complication was defined as an event that needed a specific therapy, an increased level of care, prolonged hospital stays, permanent adverse sequelae, or death. All other complications were considered minor.
Comparison of baseline data between the two groups was conducted using Student's t test for continuous variables and the chi-square test or Fisher's exact test for categorical data. The LTP, TTP, and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. Local therapeutic efficacy including technical success, technique efficacy, and LTP was assessed on a tumor basis. Intrahepatic distant recurrence, distant metastasis, TTP, and OS were evaluated on a patient basis. The following variables were analyzed as possible prognostic factors: sex, age, Child-Pugh class, etiology of liver disease, tumor size, serum alpha fetoprotein, laboratory test results (AST, ALT, bilirubin, PT), and treatment group. A p value of < 0.05 was considered statistically significant. Statistical analyses were carried out with commercially available software (SPSS version 21.0, IBM Corp., Armonk, NY, USA).
Conventional transarterial chemoembolization was successfully performed in all 85 patients in the cTACE group. In the combined group, all index tumors became radiopaque after cTACE, and were successfully targeted under US and fluoroscopic guidance. Technique efficacy was achieved in all tumors of the combined group, which was evident on CT scans taken 1 day postoperatively and showed complete replacement of index tumors by ablation area. Regarding the 1-month tumor response, complete response (CR) was observed in 83 patients (97.6%) and partial response (PR) in 2 patients (2.4%) in the cTACE group. On the other hand, CR was observed in all patients in the combined group.
The mean follow-up period was 47.2 months (range: 13–107 months) in the cTACE group and 44.1 months (range: 9–104 months) in the combined group. During the follow-up period, LTP was observed in 32 tumors of the cTACE group (30.2%) and in 15 tumors of the combined group (15.6%). The 1-, 3-, and 5-year LTP rates were 12.5%, 31.7%, and 37.0%, respectively, in the cTACE group, and 7.3%, 16.5%, and 16.5%, respectively in the combined group (p = 0.013) (Fig. 2A). Univariate and multivariate analyses revealed that cTACE monotherapy and tumor size greater than 20 mm were significant risk factors for LTP (Table 2).
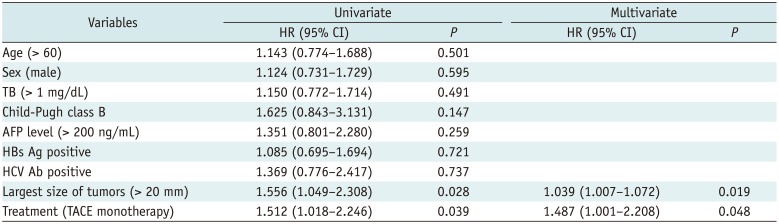
During the follow-up period, intrahepatic distant recurrence was observed in 60 patients of the cTACE group (70.1%) and in 42 patients of the combined group (51.2%). Distant metastasis occurred in 9 patients of the cTACE group (10.6%) and in 7 patients of the combined group (8.5%). The median TTP was 18 months (range: 1–98 months) in the cTACE group and 24 months (range: 1–99 months) in the combined group. The combined group showed a longer TTP compared to the cTACE group (p = 0.037) (Fig. 2B). Univariate and multivariate analyses revealed that cTACE monotherapy and the largest tumor size > 20 mm were significant risk factors for tumor recurrence (Table 3). Recurrent tumors were treated with cTACE (n = 175), RFA (n = 31), combined therapy (n = 41), hepatic resection (n = 14), liver transplantation (n = 4), and radiation therapy (n = 5). At the end of the follow-up period, 56 patients in the cTACE group (65.9%) and 57 patients in the combined group (69.5%) were alive without any viable tumors.
During the follow-up period, 7 patients in the cTACE group died of tumor progression (n = 2), hepatic failure (n = 2), and variceal bleeding (n = 3). In the combined group, there were 5 deaths caused by hepatic failure (n = 2), variceal bleeding (n = 2), and surgical complication after liver transplantation (n = 1). The cumulative OS rates at 1, 3, and 5 years were 100%, 93.2%, and 87.7%, respectively, in the cTACE group, and 100%, 96.6%, and 87.4%, respectively, in the combined group (p = 0.686) (Fig. 2C).
In the cTACE group, the changes in AST (IU/L), ALT(IU/L), TB (mg/dL), and PT (INR) values between the preoperative period and 1-month after the procedure were 1.4 ± 5.9, 1.07 ± 9.7, −0.08 ± 0.09, and −0.054 ± 0.069, respectively. In the combined group, the changes were 2.3 ± 6.6, 2.6 ± 6.9, −0.02 ± 0.1, and −0.017 ± 0.024, respectively. The changes in liver function test results were not significant in either group (p > 0.05). There was no major complication or procedure-related death in either group. Minor complications included fever (12 patients in the cTACE group and 26 in the combined group; 14.1% vs. 31.7%; p = 0.007), abdominal pain (14 and 25; 16.5% vs. 30.5% respectively; p = 0.032), and urticaria (1 patient in each group). All the minor complications were resolved with conservative treatment within 3 days.
Radiofrequency ablation is accepted as a curative treatment for HCC with minimal morbidity and mortality. However, US-guided RFA is not always feasible. Kim et al. (23) reported that US-guided percutaneous RFA was infeasible in 45 out of 136 small HCCs (33.1%). The most common reason for the infeasibility was tumor invisibility on US due to its unfavorable location, iso-echogenicity, or inability to discriminate it from surrounding cirrhotic nodules (23). New US guidance techniques such as fusion imaging are being introduced, but their limitations have not yet been completely overcome. Since the accuracy of image fusion is affected by liver displacement or deformation due to respiratory or cardiac motion, some degree of registration error between real-time US and static data collection of CT or MRI is inevitable. Furthermore, the positioning of patients during a real-time US examination can differ from that during the acquisition of reference data sets (CT or MRI). Therefore, fusion imaging with current rigid registration can cause mistargeting and incomplete ablation after RFA in small tumors. In a recent study by Song et al. (5), approximately 40% of conventional US-invisible tumors could not be ablated even after image fusion. Therefore, several studies used combined therapy using cTACE and RFA to treat tumors invisible on US (214152425). In this strategy, cTACE before RFA provides radiographic contrast to the index tumor, which facilitates targeting index tumor under fluoroscopy or CT guidance.
Previous studies using CT fluoroscopy (14) or cone-beam CT (26) to target index tumors achieved excellent technical success rates (95–100%). The present study showed that fluoroscopy with concurrent use of US also can be used as a good guiding modality. CT may be better in localization of the index tumor compared to fluoroscopy, especially when intratumoral iodized oil retention is scanty. However, RFA was performed immediately after cTACE in our study and even a small amount of iodized oil could be successfully targeted on fluoroscopy before it was washed out. Furthermore, fluoroscopic guidance has some advantages over CT guidance, when US is added appropriately. First of all, the steeply oblique approach from the lower intercostal space can be performed more conveniently under fluoroscopy than under CT. This is important in treating subphrenic tumors, in which CT-guided procedures tend to use the transthoracic approach and it is associated with frequent pulmonary complications (27). In our study, there was no serious diaphragmatic injury or pneumothorax although a substantial proportion of our patients had subphrenic tumors (47.9%). Secondly, a patient's inability to hold their breath adequately can significantly degrade the quality of the CT image due to motion artifacts. Since fluoroscopy provides real-time monitoring of respiratory movements without significant motion artifacts, the tumor with iodized oil retention can be accurately targeted without breath holding. In addition, fluoroscopy-guided procedures are presumably associated with less radiation exposure to patients and operators than CT-guided procedures (28).
Since cTACE is generally recommended in intermediate and advanced stages of HCC, there are only a few studies on the therapeutic efficacy of cTACE in early stage disease. However, according to a recent study by Kim et al. (18), the therapeutic efficacy of cTACE was acceptable in US-invisible small HCCs (5-year survival of 74.5%). Thus, even though the combined therapy may improve technical feasibility of RFA in US-invisible HCCs, the therapeutic benefit over cTACE monotherapy should be proven to support this strategy. However, there are few comparative studies addressing this issue. Hyun et al. (26) demonstrated additional gain of combined therapy over cTACE in 91 patients with US-invisible early stage HCCs in terms of TTP (mean 29.7 vs. 34.9 months) and OS (3-year OS: 71% vs. 93%). However, this study has an inherent limitation in that combined therapy was performed more recently and compared with historical cTACE data (26). The present study compared the two treatments performed within the same period minimizing the potential bias and included a larger population and longer follow-up. This study showed that combined therapy provided better local tumor control (5-year LTP: 37% vs. 16.5%) and TTP (mean 35.8 months vs. 47.4 months) compared with cTACE monotherapy. Multivariate analysis showed that combined therapy was a significant independent factor for better local tumor control and longer TTP. This result suggests that additional RFA is beneficial when US-guided RFA infeasible tumors become RFA-feasible after cTACE.
Although the combined group showed better LTP and TTP than the cTACE group, there was no difference in OS between the two groups. OS can be influenced by many factors such as the progression of liver cirrhosis, treatment for recurrent tumors, and comorbidities. Therefore, better local tumor control might not result in better OS. In addition, the OS of the cTACE group (1-, 3-, 5-year OS: 100%, 93.2% and 87.7%) seems relatively higher compared to those reported in previous studies. For example, Kim et al. (18) reported 1-, 3-, and 5-year OS rates as 97.6%, 86.7%, and 74.5%, respectively after cTACE in patients with RFA-infeasible small HCCs (n = 122). Hyun et al. (17) reported 1-, 2-, and 3-year survival rates after cTACE in the same clinical setting as 91%, 79%, and 71%, respectively. The favorable OS of the cTACE group in our study may be due to aggressive multimodal treatments for recurrent tumors. As a result, 56 patients in the cTACE group (65.9%) were alive without a viable tumor at the end of the follow-up period, which was similar to that of the combined group (69.5%). This suggests that subsequent treatments for recurrent tumors are as important as the first line therapy for a patients' OS.
This study showed that tumor size as well as treatment modality were significant risk factors for LTP and TTP. This result is not unexpected because the size of a tumor is a well-known risk factor for tumor progression after most locoregional treatments (2930). Many previous studies classified all small tumors less than 3 cm in one group (1731). However, the results from our study suggest that there is a difference between tumors less than 2 cm and tumor of 2–3 cm in terms of LTP and TTP. Thus, in future studies, small tumors need to be further subdivided based on size to elucidate the association between tumor size and tumor progression.
In the locoregional therapy for HCCs, it is crucial to preserve post-treatment liver functions (32). In early studies regarding combined therapy, RFA was performed 1–2 weeks after cTACE to gain time for functional recovery of the liver (33). However, theoretically, it is desirable to reduce the time interval between cTACE and RFA so that the effectiveness of combined therapy is maximized (3435). Even though all the RFA procedures were performed immediately after cTACE in this study, there was no significant change between pre- and post-procedural liver function test in the combined group. Some minor complications such as fever and abdominal pain were more frequently found in the combined group. An interventional radiologist should be aware of this and inform the patients before the procedure. However, since these minor complications were resolved in a few days without any specific treatment, additional RFA after cTACE in small HCCs is unlikely to be seriously harmful for patients' safety or interfere with future treatment plans.
This study has several limitations, which are largely due to its retrospective design. First, the treatment option was chosen depending on patients' preference, which in practice, could be influenced by the operators' preference as well. This may potentially cause selection bias. For example, the operator might be reluctant to perform combined therapy depending on the intrahepatic tumor location and instead encourage the patient to receive cTACE by providing biased information. However, there was no difference in RFA-unfavorable location of tumors between the two groups. Second, a considerable number of patients received various treatments for recurred tumors during the follow-up period, which makes it difficult to assess the therapeutic impact of first line therapy on clinical outcomes. However, this problem is unavoidable as there is no definite guideline for such cases and controlling treatment options for recurred tumors is not practical even in a prospective study. Third, this study did not employ a US-CT/MRI fusion imaging technique, which has been proven to be useful in the treatment of US-invisible HCCs. Although this study demonstrated the feasibility of fluoroscopy-guided RFA, comparison of combined therapy using fluoroscopic guidance of RFA using fusion imaging techniques is needed to thoroughly investigate the strengths and weaknesses of each treatment.
In conclusion, combined therapy using cTACE followed by fluoroscopy-guided RFA is a safe and effective treatment of US-invisible early stage HCC (BCLC stage 0 or A). This strategy provides less LTP and longer TTP than cTACE monotherapy.
References
1. Rhim H, Lee MH, Kim YS, Choi D, Lee WJ, Lim HK. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol. 2008; 190:1324–1330. PMID: 18430851.

2. Kim J, Yoon CJ, Seong NJ, Jeong SH, Kim JW. Fluoroscopy-guided radiofrequency ablation for small hepatocellular carcinoma: a retrospective comparison with ultrasound-guided ablation. Clin Radiol. 2015; 70:1009–1015. PMID: 26126713.

3. Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, et al. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol. 2009; 20:490–499. PMID: 19328427.

4. Lee MW, Rhim H, Cha DI, Kim YJ, Choi D, Kim YS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma: fusion imaging guidance for management of lesions with poor conspicuity at conventional sonography. AJR Am J Roentgenol. 2012; 198:1438–1444. PMID: 22623560.

5. Song KD, Lee MW, Rhim H, Cha DI, Chong Y, Lim HK. Fusion imaging-guided radiofrequency ablation for hepatocellular carcinomas not visible on conventional ultrasound. AJR Am J Roentgenol. 2013; 201:1141–1147. PMID: 24147489.

6. Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, et al. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012; 81:1173–1178. PMID: 21466931.

7. Peng ZW, Chen MS. Transcatheter arterial chemoembolization combined with radiofrequency ablation for the treatment of hepatocellular carcinoma. Oncology. 2013; 84(suppl 1):40–43. PMID: 23428857.

8. Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013; 269:927–937. PMID: 24086071.

9. Wang X, Hu Y, Ren M, Lu X, Lu G, He S. Efficacy and safety of radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinomas compared with radiofrequency ablation alone: a time-to-event meta-analysis. Korean J Radiol. 2016; 17:93–102. PMID: 26798221.

10. Zheng L, Li HL, Guo CY, Luo SX. Comparison of the efficacy and prognostic factors of transarterial chemoembolization plus microwave ablation versus transarterial chemoembolization alone in patients with a large solitary or multinodular hepatocellular carcinomas. Korean J Radiol. 2018; 19:237–246. PMID: 29520181.

11. Zhu ZX, Liao MH, Wang XX, Huang JW. Transcatheter arterial chemoembolization plus 131I-labelled metuximab versus transcatheter arterial chemoembolization alone in intermediate/advanced stage hepatocellular carcinoma: a systematic review and meta-analysis. Korean J Radiol. 2016; 17:882–892. PMID: 27833404.
12. Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013; 19:3872–3882. PMID: 23840128.

13. Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009; 252:905–913. PMID: 19567647.

14. Takaki H, Yamakado K, Nakatsuka A, Yamada T, Uraki J, Kashima M, et al. Computed tomography fluoroscopy-guided radiofrequency ablation following intra-arterial iodized-oil injection for hepatocellular carcinomas invisible on ultrasonographic images. Int J Clin Oncol. 2013; 18:46–53. PMID: 22016114.

15. Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol. 2009; 82:908–915. PMID: 19433482.

16. Lee MW, Kim YJ, Park SW, Jeon HJ, Yi JG, Choe WH, et al. Percutaneous radiofrequency ablation of liver dome hepatocellular carcinoma invisible on ultrasonography: a new targeting strategy. Br J Radiol. 2008; 81:e130–e134. PMID: 18440934.

17. Hyun D, Cho SK, Shin SW, Park KB, Park HS, Choo SW, et al. Early stage hepatocellular carcinomas not feasible for ultrasound-guided radiofrequency ablation: comparison of transarterial chemoembolization alone and combined therapy with transarterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol. 2016; 39:417–425. PMID: 26246215.

18. Kim JW, Kim JH, Sung KB, Ko HK, Shin JH, Kim PN, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014; 109:1234–1240. PMID: 24935276.
19. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.

20. Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, et al. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012; 23:287–294. PMID: 22284821.

21. Ahmed M. Technology Assessment Committee of the Society of Interventional Radiology. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014; 25:1706–1708. PMID: 25442133.

22. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60. PMID: 20175033.

23. Kim JE, Kim YS, Rhim H, Lim HK, Lee MW, Choi D, et al. Outcomes of patients with hepatocellular carcinoma referred for percutaneous radiofrequency ablation at a tertiary center: analysis focused on the feasibility with the use of ultrasonography guidance. Eur J Radiol. 2011; 79:e80–e84. PMID: 21514757.

24. Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, et al. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011; 84:691–697. PMID: 21750136.

25. Gandhi S, Iannitti DA, Mayo-Smith WW, Dupuy DE. Technical report: lipiodol-guided computed tomography for radiofrequency ablation of hepatocellular carcinoma. Clin Radiol. 2006; 61:888–891. PMID: 16978986.
26. Hyun D, Cho SK, Shin SW, Rhim H, Koh KC, Paik SW. Treatment of small hepatocellular carcinoma (≤ 2 cm) in the caudate lobe with sequential transcatheter arterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol. 2016; 39:1015–1022. PMID: 26975761.
27. Yamakado K, Nakatsuka A, Takaki H, Sakurai H, Isaji S, Yamamoto N, et al. Subphrenic versus nonsubphrenic hepatocellular carcinoma: combined therapy with chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2010; 194:530–535. PMID: 20093620.

28. Schulz B, Heidenreich R, Heidenreich M, Eichler K, Thalhammer A, Naeem NN, et al. Radiation exposure to operating staff during rotational flat-panel angiography and C-arm cone beam computed tomography (CT) applications. Eur J Radiol. 2012; 81:4138–4142. PMID: 22304981.

29. Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003; 97:1253–1262. PMID: 12599233.

30. Yang B, Zou J, Xia J, Ren Z, Gan Y, Wang Y, et al. Risk factors for recurrence of small hepatocellular carcinoma after long-term follow-up of percutaneous radiofrequency ablation. Eur J Radiol. 2011; 79:196–200. PMID: 20303686.

31. Song MJ, Bae SH, Lee JS, Lee SW, Song DS, You CR, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016; 31:242–252. PMID: 26874512.

32. Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J Gastroenterol. 2016; 22:1935–1942. PMID: 26877601.

33. Kuroda H, Kasai K, Kakisaka K, Yasumi Y, Kataoka K, Ushio A, et al. Changes in liver function parameters after percutaneous radiofrequency ablation therapy in patients with hepatocellular carcinoma. Hepatol Res. 2010; 40:550–554. PMID: 20546330.

34. Wang ZJ, Wang MQ, Duan F, Song P, Liu FY, Chang ZF, et al. Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol. 2013; 19:4192–4199. PMID: 23864783.

35. Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000; 217:119–126. PMID: 11012432.

Fig. 1
66-year-old man who underwent combined therapy for single early hepatocellular carcinoma.
A, B. Contrast-enhanced magnetic resonance image shows 2.3-cm bilobed tumor (arrows) with hypervascularity in arterial phase (A) and washout in delayed phase (B) in right hepatic dome. C. Radiograph obtained during fluoroscopy-guided radiofrequency ablation shows expandable radiofrequency electrode accurately positioned at index tumor with iodized oil retention (arrow) induced by cTACE. D. One-day follow-up CT scan shows low attenuating ablation area (arrow) completely surrounding index tumor with iodized oil. E. Follow-up CT scan obtained 4 years after combined therapy shows shrinkage of index tumor without LTP (arrow). CT = computed tomography, cTACE = conventional transarterial chemoembolization, LTP = local tumor progression
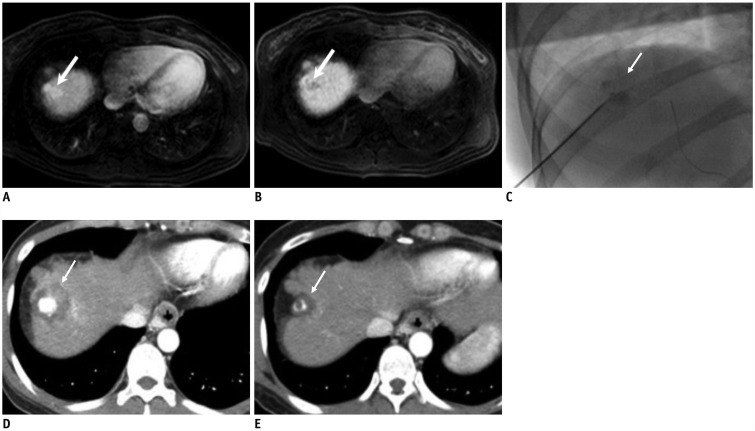
Fig. 2
Graphs showing LTP (A), time to progression (B), and OS (C) stratified by treatment group.
OS = overall survival
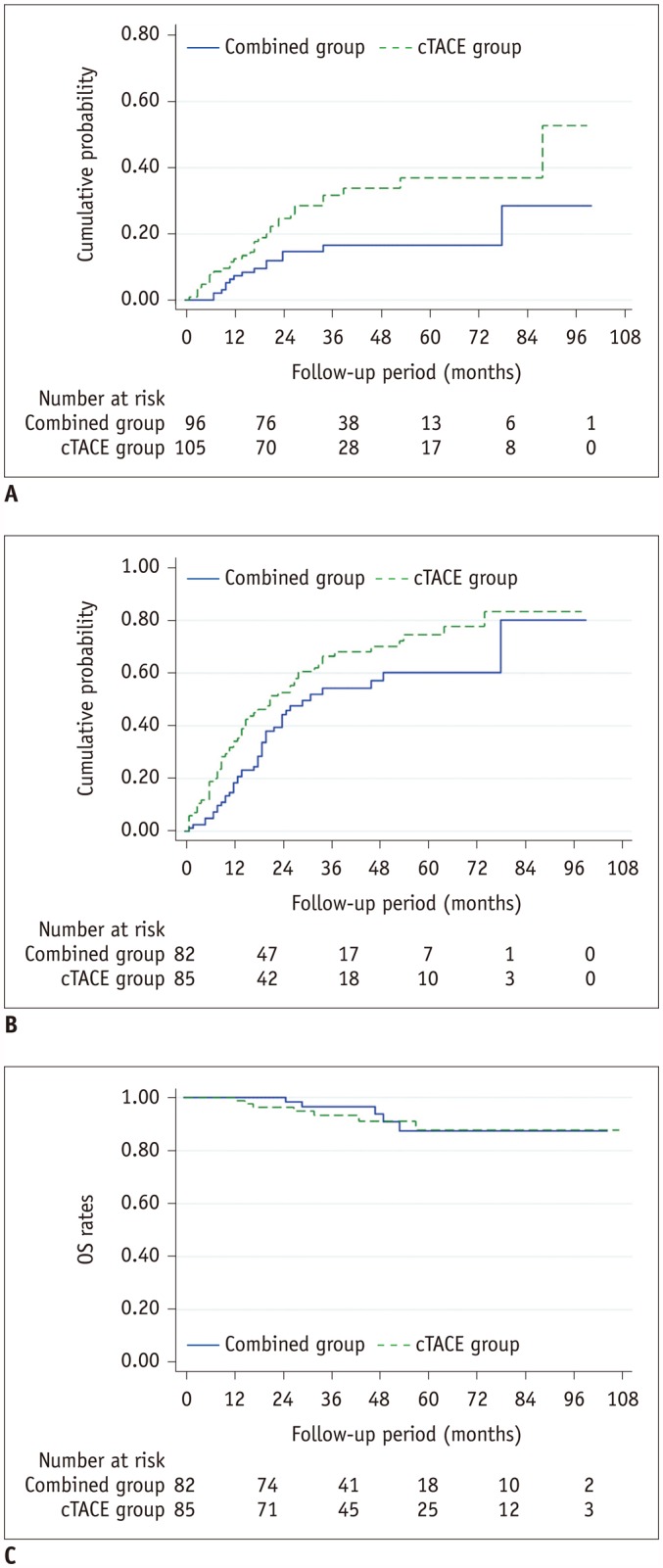
Table 1
Baseline Patient and Tumor Characteristics
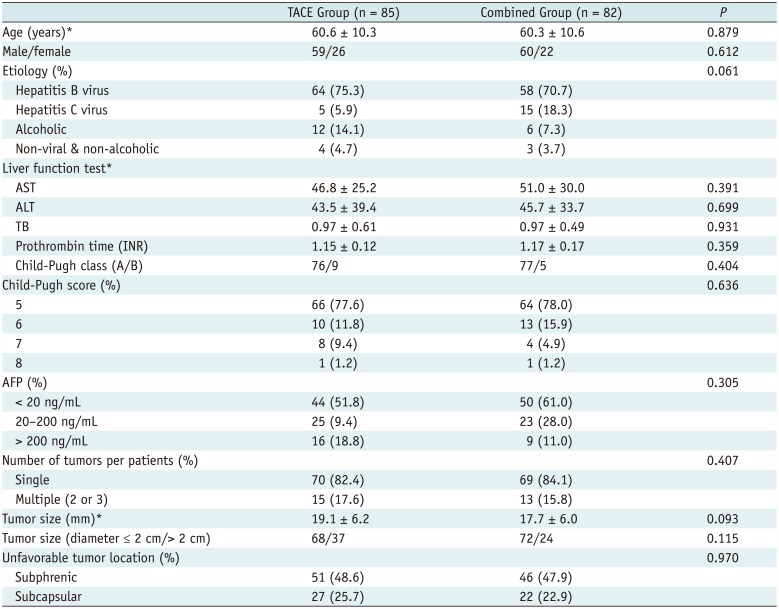
Table 2
Univariate and Multivariate Analyses of Risk Factors for Local Tumor Progression
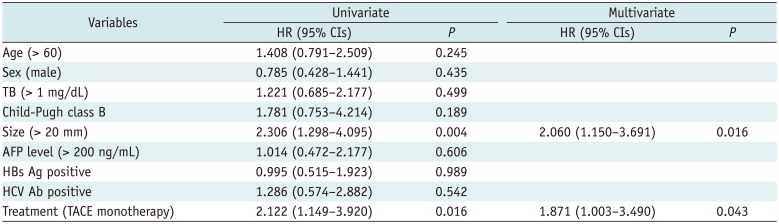
Table 3
Univariate and Multivariate Analyses of Risk Factors for Time to Progression




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download