INTRODUCTION
MATERIALS AND METHODS
Derivation Study for DECTE Index
Patient Data
Clinical Parameters for CD Activity
CT Scanning
CT Image Analysis and Iodine Quantification
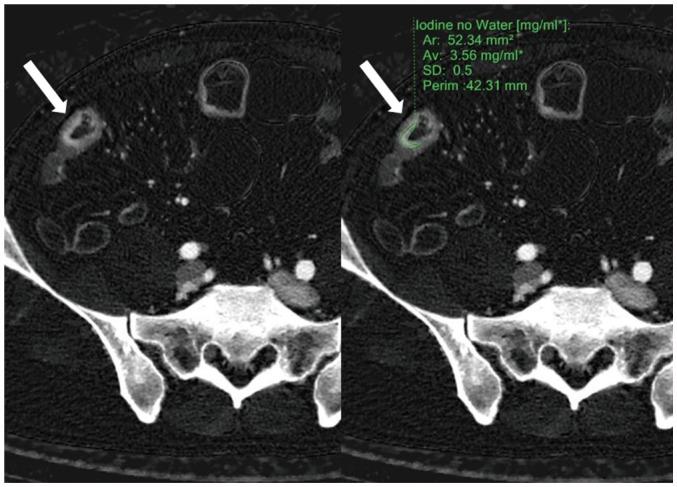 | Fig. 2ROI application.Radiologists applied ROI (green line, right image) to diseased bowel wall (arrows) that showed strongest enhancement in most conspicuous part of small and large bowels on iodine concentration maps generated from enteric phase CT. In this particular patient, iodine concentration measured 3.56 mg·I/mL. Ar = area, Av= average, ROI = region-of-interest, SD = standard deviation
|
Statistical Analysis
Validation Study for DECTE Index
RESULTS
Derivation Study
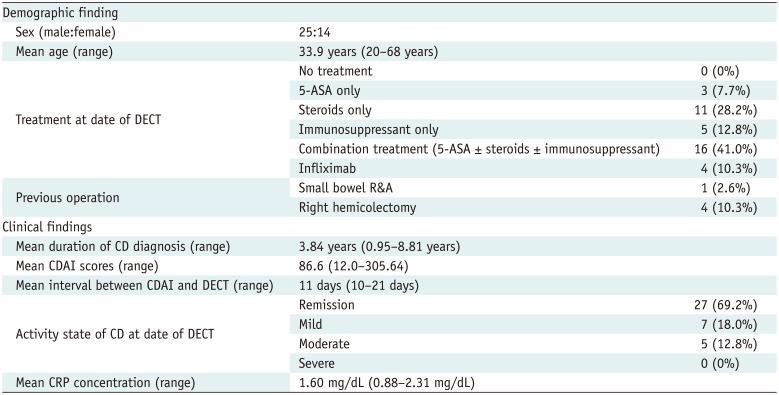
Clinical Findings
Table 1
Demographic and Clinical Characteristics of 39 Patients in Derivation Study
Radiation Dose
DECTE Findings
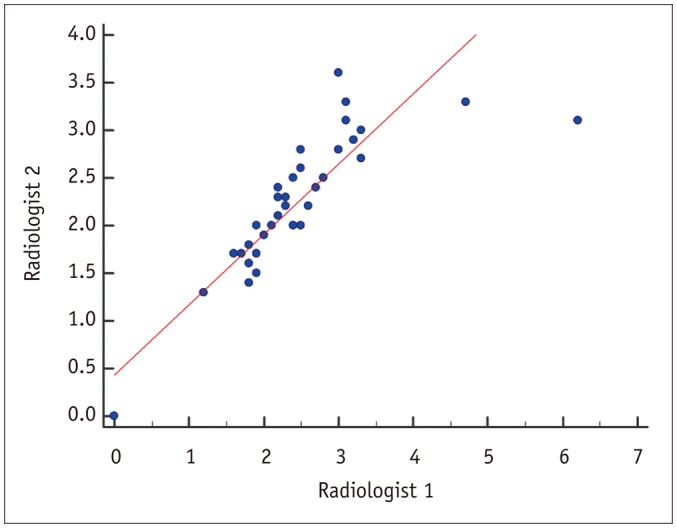 | Fig. 3Scatter diagram of iodine quantification by two radiologists.Inter-reader agreement was very good, with correlation coefficient of 0.838 (p < 0.001).
|
Table 2
Results of Spectral Detector-Based DECTE Findings
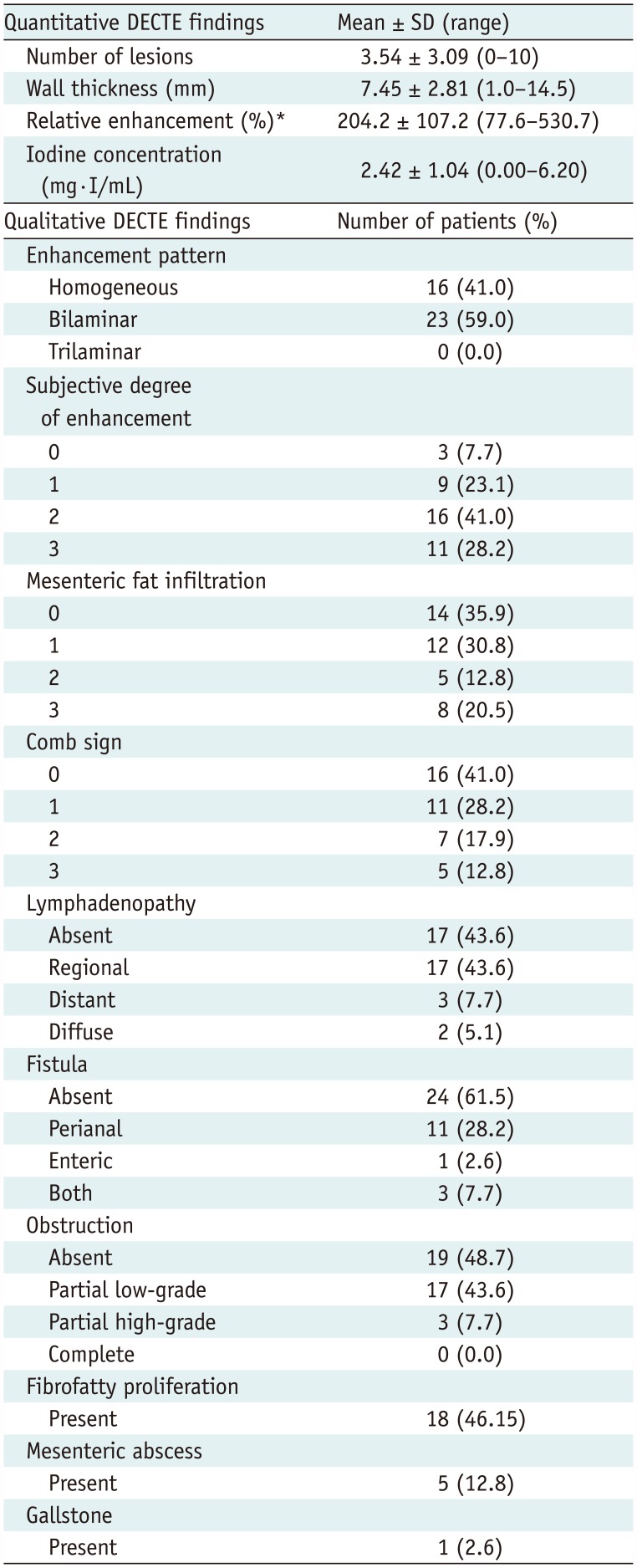
Table 3
Statistical Results of DECTE Features between Remission and Active Groups in Derivation Study
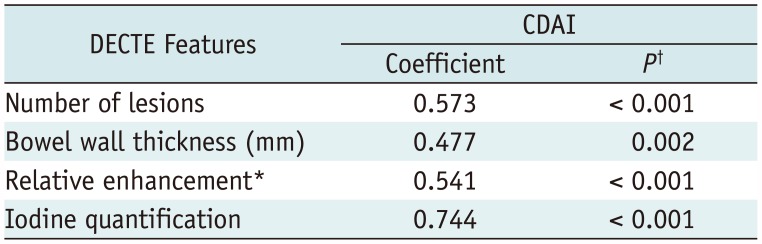
DECTE Findings and CDAI
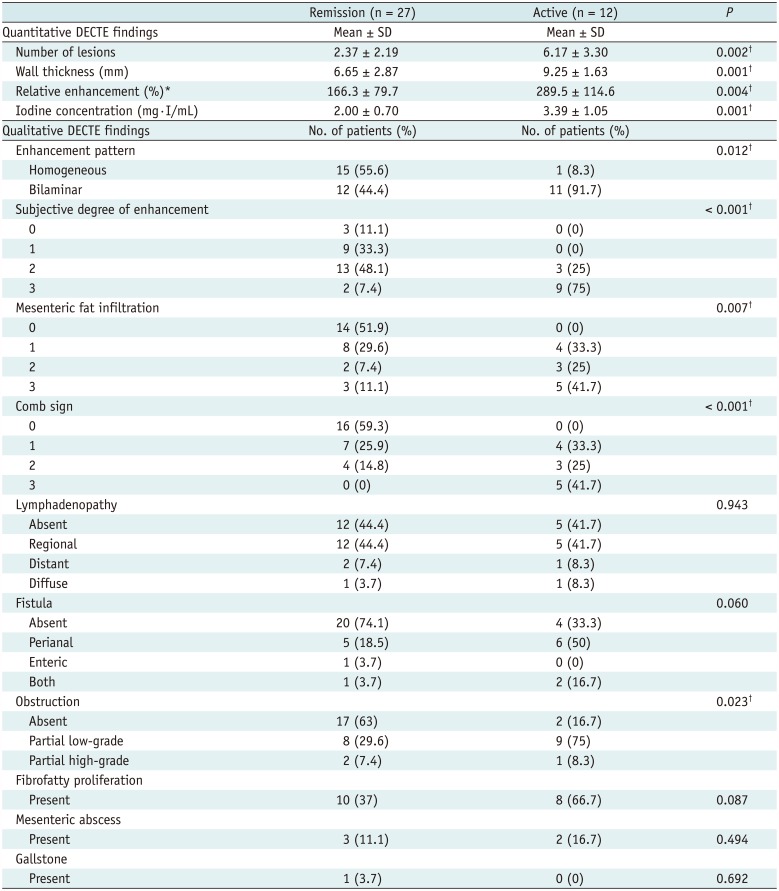
 | Fig. 428-year-old male with known CD.
A. On DECTE enteric-phase image, subtly-enhancing eccentric wall thickening (arrow) is visible in distal ileum. Relative enhancement of this lesion measured 131%. B. On iodine map of DECTE, iodine concentration of lesion (arrow) was 1.6 mg·I/mL. Therefore, estimated CDAI with linear regression equation (CDAI = 53.549 × iodine concentration + 55.111) was 141, which was score for remission (CDAI < 150). Patient's real CDAI score was 24, which also indicated remission state.
|
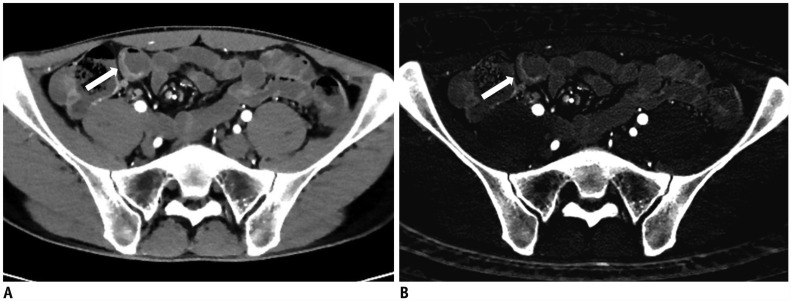
 | Fig. 541-year-old female with known CD.
A. On DECTE enteric-phase image, focal enhancing and concentric wall thickening (arrow) is visible in terminal ileum. Relative enhancement of this lesion was 201%. B. On iodine map of DECTE, iodine concentration of lesion (arrow) measured 2.5 mg·I/mL. Therefore, estimated CDAI with linear regression equation (CDAI = 53.549 × iodine concentration + 55.111) was 189.0, which was score for mild activity (CDAI: 150–219). Patient's real CDAI score was 164, which also indicated mildly active state of CD.
|
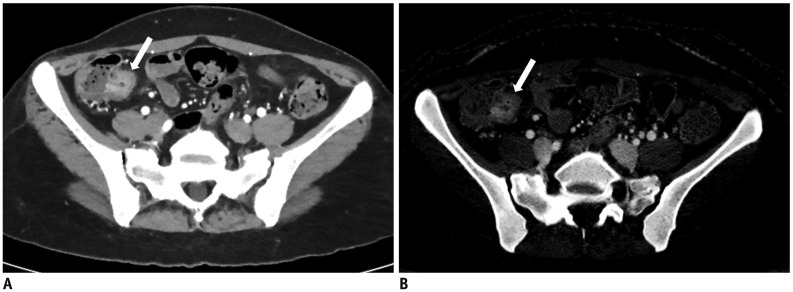
 | Fig. 617-year-old female with known CD.
A. On DECTE enteric-phase image, focal enhancing and eccentric wall thickening (arrow) is clearly visible in distal ileum. Relative enhancement of this lesion was 310%. B. On iodine map of DECTE, iodine concentration of lesion (arrow) measured 3.3 mgI/mL. Therefore, estimated CDAI with linear regression equation (CDAI = 53.549 × iodine concentration + 55.111) was 231.8, which was score for moderate activity (CDAI: 220–450). Patient's real CDAI score was 262, which also indicated moderately active state of CD.
|
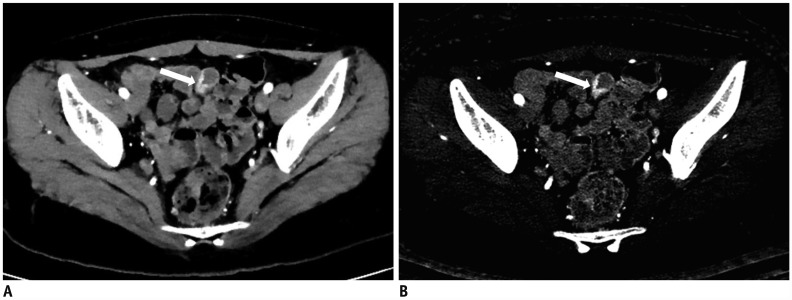
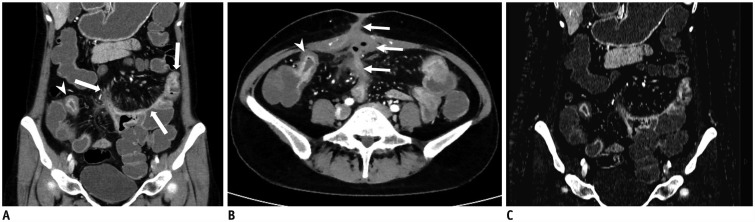 | Fig. 736-year-old female with known CD.
A, B. On coronal (A) and axial (B) images of DECTE obtained during enteric phase, multifocal, asymmetrically enhanced bowel wall thickenings (arrows) are prominently visible in ileum. Bilaminar pattern of enhancement (arrowheads) is also demonstrated on involved bowel. Note enterocutaneous fistula (thin arrows) between distal ileum and periumbilical area. Comb signs showing mild venous dilation (grade 2) and severe perienteric fat infiltration (grade 3) were also depicted. Relative enhancement of lesion showing strongest enhancement was 247%. C. On iodine map of DECTE, iodine concentration of involved bowel wall measured 4.7 mg·I/mL. Therefore, estimated CDAI with linear regression equation (CDAI = 53.549 × iodine concentration + 55.111) was 306.8, which was score for moderate activity (CDAI: 220–450). Patient's real CDAI score was 305, which also indicated moderately active state of CD.
|
Multiple Linear Regression Analysis
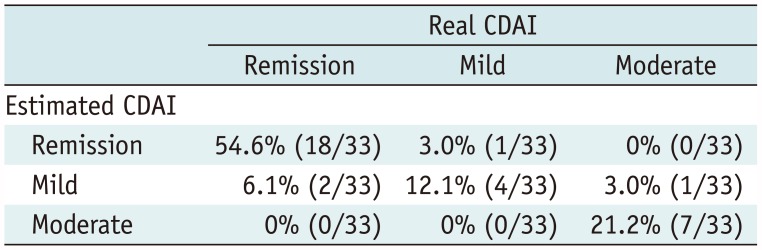
Validation Study
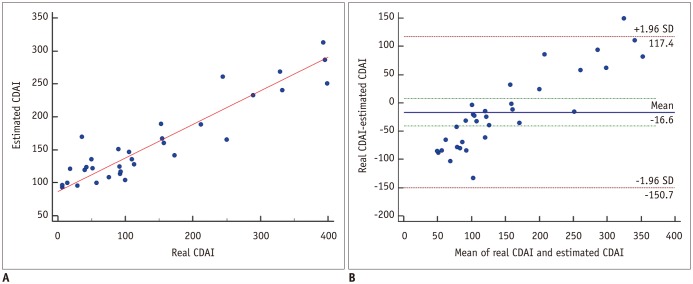 | Fig. 8Scatter-plots between real and estimated CDAI in 33 validation patients.
A. On Pearson's correlation test, there was significant correlation (r = 0.925) between real and estimated CDAI scores (p < 0.001). B. In Bland-Altman test, these scoring methods had mean measurement bias of −16.6 and limits of agreement ranging from −150.7 to 117.4.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download