Abstract
Synovial sarcoma is a distinct and generally recognized soft tissue tumor that it’s origin still raises controversy. The synovial origin of synovial sarcoma has not been determined despite the accepted terminology implying synovium as stem cell. Three cases of primary synovial sarcoma (2 fibrous monophasic, 1 biphasic type) were studied with a panel of antibodies against different types of cytokeratin and other markers (EMA, CEA, vimentin, S-100 protein, lysozyme, 1-antichymotrypsin). Spindle shaped-cell in monophasic synovial sarcoma showed reactivity for CK7 and pancytokeratin. Epithelial cells lining of glands in biphasic synovial sarcoma reactive for CK7, pancytokeratin, EMA, and foe ally CEA but spindle cells only positive for vimentin. By electron microscopy, fibrous monophasic synovial sarcoma showed pseudogland formation with intercellular junctions of paired subplasmalemmal density and discontinuous basal lamina. These results indicate that synovial sarcoma showes epithelial differentiation. We believe that synovial sarcoma arises in pluripotential connective tissue cells that is able to be differentiated into both mesenchymal and epithelial components. So, synovial sarcoma have been considered carcinosarcoma of soft tissues depending on the type of differentiation.
REFERENCES
1. Abenoza P, Manivel JC, Swanson PE, Wick MR. Synovoal sarcoma, Ultrastructural study and immunohistochemical analysis by a combined per-oxidase-abtiperoxidase/avidinbiotin peroxidase comples procedure. Hum Pathol. 1986; 17:1107–1115.
2. Corson JM, Weiss LM, Banks-Schlegel SP, Pinkus GS. Keratin proteins and carcinoembronic antigen in synovial sarcomas: An immunohistochemical study of 24 cases. Hum Pathol. 1984; 15:615–621.
3. Weiss SW. Synovial sarcoma. In Soft tissue tumors. 1994. 3nd ed. St. Louis: Mosby;p. pp 757–786.
4. Krall RA, Kostianovsky M, patchefsky AS. Synovial sarcoma. A clinical, pathological, and ultrastructural study of 26 cases supporting the recognition of a monophasic variant Am. J Surg Pathol. 1981; 5:137–151.
5. Lopes JM, Bjerkehagen B, Sobrinoho-Simoes M, Nesland JM. The ultrastructural spectrum of synovial sarcomas. A study of the epithelial type differentition of primay tumors, recurrences, and metastases. Ultrastruct Pathol. 1993; 17:137–151.
6. Miettien M, Virtanen I. Synovial sarcoma-a misnomer. Am J Pathol. 1984; 117:18–25.
7. Mukai M, Torikata C, Iri H, Hanaoka H, Kawai T, Yakumaru K, Shimoda T, Mikata A. Kageyama : Cellular differentiation of epithelioid sarcoma. An electronmicroscopic, enzyme-histochemical, and immunohistochemical study. Am J Pathol. 1985; 119:44–56.
8. Otdonez NG, Mahfouz SM, Mackay B. Synovial sarcoma. An immunohistochemical and ultrastructural study. Hum Pathol. 1990; 21:733–749.
9. Quinonez G, simon GT. Cellular junctions in a spectrum of human malignant tumors. Ultrastruct Pathol. 1988; 12:389–405.

10. Ramaekers FCS, Huysmans A, Schaart G; Moesker and Voojis GP. Tissue distribution of keratin 7 as monitored by a monoclonal antihbody. Exp Cell Res. 1987; 170:235–249.
11. Russell WO, Cohen J, Enzinger F, Handu SI, Heise H, Martin RG, Meissner W, Miller WT, Schmitz RL, Suit HD. A clinical and pathological staging system for soft tissue sarcomas. Cancer,. 1977; 40:1562–1570.

12. Salisbury JR, Isaacson PG. Synovial sarcoma. An immunohistochemical study. J Pathol. 1985; 147:49–57.

13. Sumitomo M, Hirose T, Kudo E, Sano T, Shinomiya S, Hizawa K. Epithelial differentition in synovial sarcoma. Correlation with histology and immunophenotypic expression. Acta Pathol Jpn. 1989; 39:381–387.
Fig. 1
On enhanced T1-weighted image, heterogenous enhanced soft tissue mass shows multilobulated contour with internal septation at left gluteus muscle.
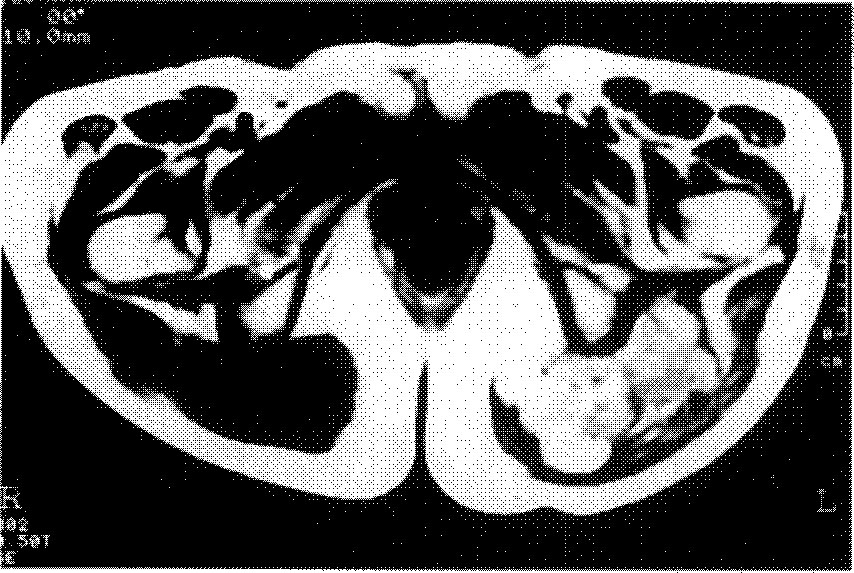
Fig. 2
On chest PA, multiple, variable sized hematogenous metastatic nodules ard scattered in the entire lung, and also noted the right pleural effusion.

Fig. 3-A
Monophasic fibrous synovial sarcoma, Tightly apposed spindle cells reveals fibrosarcoma like pattern (H&E, x100). B. Plump spindle cells are reactive for vimentin in case 1 (ABC, x200). C. Diffusely packed fibroblastlike spindle cells reactive for pankeratin in case 2 (ABC, x100).
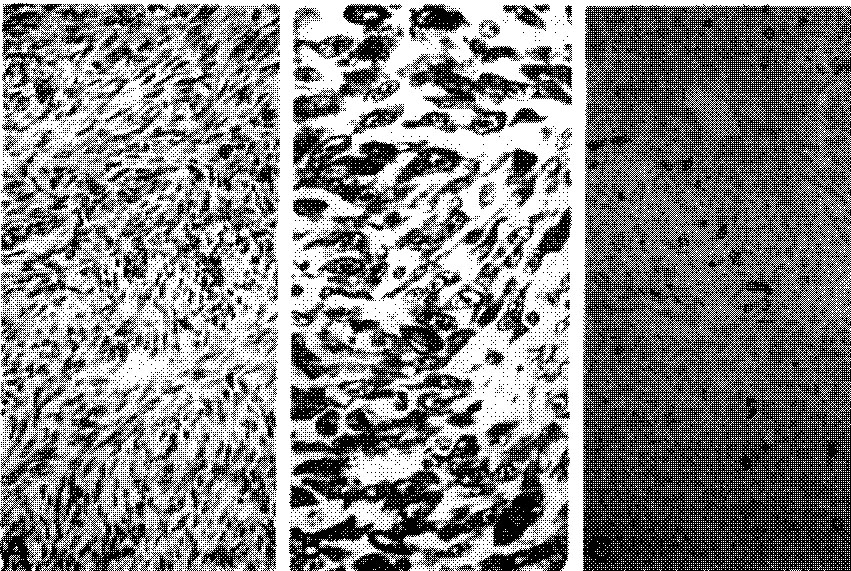
Fig. 4
Paired subplasmalemmal density(PSD, arrow) is observed between tightly packed spindle cells (Uranyl acetate and lead citrate, Original magnification x2,800).

Fig. 5
PSD(arrow) and discontinuous basal lamina around spindle cells are observed (Uranyl acetate and lead citrate, Original magnification x2,800).

Fig. 6
Spindle cells showing pseudoglandular formation are observed.(Uranyl acetate and lead citrate, Original magnification x2,800).

Table 1
Monoclonal Polyclonal Antibodies Used in This Study
Table 2
Clinical Data and Histologic Type
| Case | Sex | Age | Location | Type |
|---|---|---|---|---|
| 1 | F | 37 | Thigh, left | Monophgasic spindle type |
| 2 | F | 34 | Buttock, left | Monophgasic spindle type lung* |
| 3 | F | 37 | Ingunal, right | Biphasic type |
Table 3
Results of the Immunohistochemical Stain of 3 Cases of Synovial Sarcoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download