Abstract
Intravenous thrombolysis (IVT) and endovascular treatment (EVT) are currently the main treatments for reperfusion in acute ischemic stroke. Although the EVT recanalization rate has increased, unsuccessful recanalization is still observed in 10-30% cases. Superficial temporal artery-middle cerebral artery (STA-MCA) bypass is considered a rescue therapy in such cases, but in most centers it is not usually performed for acute ischemic stroke. Graft occlusion is rare following STA-MCA bypass, but it might lead to recurrent ischemic stroke. We hereby report on a patient with right MCA infarction and in whom EVT failed due to complete proximal internal carotid artery occlusion. He underwent an emergency STA-MCA bypass, resulting in a full recovery of his motor weakness. However, six months later, the patient experienced recurrent acute ischemic stroke due to bypass graft occlusion. His EVT failed again but revision bypass surgery, using STA remnant branch, was successful with full motor weakness recovery. We recommend a revision bypass surgery as a feasible therapeutic option for recurrent cerebral infarction caused by delayed STA graft occlusion.
In Korea, the incidence rate of ischemic stroke increases annually due to the increase in the aging population and lifestyle westernization.7) Reperfusion therapy is important for the treatment of acute ischemic stroke with intravenous thrombolysis (IVT) and endovascular treatment (EVT) being the main current therapeutic options. If IVT or EVT is not available, bypass surgery could be considered as an alternative reperfusion therapy. Excellent graft patency has been reported after superficial temporal artery-middle cerebral artery (STA-MCA) bypass.6) However, delayed bypass graft occlusion might cause recurrent ischemic stroke after several months or even years after the procedure. We hereby report a rare case of a patient who underwent revision STA-MCA bypass 6 months after initial bypass surgery due to recurrent ischemic stroke caused by bypass graft occlusion.
A 46-year-old man presented with mild left hemiparesis (motor grade IV/IV) and mild dysarthria. Diffusion-weighted imaging (DWI) revealed acute ischemic lesions in multiple areas of the right hemisphere (Fig. 1A). Magnetic resonance angiography revealed right MCA and internal carotid artery (ICA) occlusions (Fig. 1B), while perfusion-weighted imaging (PWI) demonstrated considerable defects in the right MCA territory (Fig. 1C). The patient presented with fluctuating symptoms and gradual neurologic worsening for 2 days, despite receiving maximal medical treatment. His left side motor function deteriorated from grade IV to grade III and his aphasia and dysarthria were aggravated. The patient was referred to a neurointerventional team and underwent EVT, which failed because of complete right proximal ICA occlusion. An emergent STA-MCA bypass using a parietal branch of the STA was performed and clopidogrel, at 75 mg/day, was prescribed 1 day after surgery. Postoperative day 7 DWI revealed no interval changes in the acute infarct lesion (Fig. 1D), while PWI demonstrated a markedly improved perfusion status in the right MCA territory (Fig. 1E). Digital subtraction angiography (DSA) on postoperative day 18 showed good bypass graft patency (Fig. 1F). The patient's neurological symptoms gradually improved and his motor weakness recovered fully.
Six months later, the patient was admitted to our emergency room due to sudden left hemiparesis (motor grade IV/IV). Magnetic resonance imaging (MRI) revealed acute ischemic lesions in multiple areas of the right hemisphere during DWI (Fig. 2A) and considerable perfusion defects in the right MCA territory on PWI (Fig. 2B). Emergency DSA revealed STA bypass graft occlusion (Fig. 2C) and 88% left cervical ICA stenosis (Fig. 2D). We considered a revision bypass surgery difficult due to postoperative adhesions, and we performed carotid artery stenting (CAS) of the left cervical ICA to increase blood flow via the anterior communicating artery (AcomA) into the right MCA territory. Immediately after CAS, blood flow via AcomA into the right MCA territory increased (Fig. 2E). The patient received a dual loading of aspirin/clopidogrel antiplatelet therapy and induced hypertension therapy. However, one day after CAS, his left side motor weakness deteriorated from grade IV to grade III despite maximal medical treatment, and his follow-up MRI revealed increased cerebral infarction on DWI (Fig. 2F) and large perfusion defects in right MCA territory on PWI (Fig. 2G). To prevent further progression of the ischemic stroke, emergency revision bypass surgery was performed. The initial craniotomy site was reopened, and the STA frontal branch was connected to another M4 segment by end-to-side anastomosis (Fig. 3A). The patient was started on cilostazol 200 mg/day one day after surgery, and aspirin 100 mg/day was added seven days later. Postoperative day 7 PWI demonstrated improved perfusion in the right MCA territory (Fig. 3B), and DWI revealed a slight increase in cerebral infarction on the remnant perfusion defect area (Fig. 3C). After surgery, the patient's motor weakness was recovered fully. Follow-up cerebral angiography showed excellent bypass graft patency 3 months after the revision bypass surgery (Fig. 3D). At the 3-year follow-up visit, the patient remained well and continued to take cilostazol and aspirin.
In the acute stage of ischemic stroke, IVT and EVT are the main current therapeutic options for reperfusion therapy. Although the EVT recanalization rate has increased, unsuccessful recanalization is still observed in 10–30% cases.1)5)9)12) In such cases, carotid endarterectomy (CEA) and STA-MCA bypass surgery can be considered as alternative reperfusion therapies. CEA is suitable for treating ICA stenosis but not for total ICA occlusion. STA-MCA bypass surgery is not usually performed for acute ischemic stroke in most centers. STA-MCA bypass surgery is generally used as an elective surgery for stroke prevention in chronic cerebrovascular insufficiencies, such as Moyamoya disease and atherosclerotic occlusive disease. Nevertheless, there have been some recent reports supporting the use of STA-MCA bypass as a treatment option in selected patients with acute ischemic stroke.8)11) After STA-MCA bypass, graft STA occlusion is known to be rare and Halsey et al.6) reported a high rate of bypass graft patency (approximately 96%). However, delayed bypass graft occlusion might occur and can result in recurrent cerebral infarction. Supplying the broad MCA territory via an STA graft for a long period causes hemodynamic stress on the STA vessel wall, which in turn leads to atherosclerotic changes. In our case, delayed bypass graft occlusion caused a recurrent cerebral infarction six months after initial STA-MCA bypass surgery, and the patient was successfully treated with revision bypass surgery.
Because we considered revision bypass surgery difficult to be performed due to postoperative adhesions, the patient initially underwent CAS of the contralateral left ICA to increase right MCA blood flow through AcomA. However, his symptoms aggravated after CAS and follow-up MRI revealed an increase in cerebral infarction and still large perfusion defects in the right MCA territory. Although, ipsilateral cerebral infarction resulting from embolic events after CAS is common, contralateral cerebral infarction is uncommon and the pathogenic mechanism behind this is unclear. We hypothesize that the right MCA cerebral infarction increase following left ICA CAS resulted from hemodynamic changes in cerebral blood flow. After left ICA stenting, increased blood flow from the left ICA to the left MCA might lead to decreased blood flow to the right cerebral hemisphere. Contralateral cerebral infarction occurring after unilateral carotid angioplasty with stenting has been previously reported, with the authors also suggesting postoperative hemodynamic changes as the cause.10) It should be noted that caution is needed when performing CAS for the increase of blood flow in the contralateral hemisphere via AcomA.
During STA-MCA bypass surgery, neurosurgeons generally use the parietal STA branch and some neurosurgeons tie the frontal STA branch to increase blood flow to the bypass graft. However, Duckworth et al.4) measured and compared STA branch blood flow before and after clipping one of the STA branches, and did not find any significant differences. We suggest that it would be better to preserve the unused STA branch in case of delayed STA graft occlusion. In our case, the STA parietal branch bypass graft was tapered probably due to the delayed occlusion and we used the remnant STA frontal branch that seemed thickened in the preoperative DSA, as compared to the previous DSA six months earlier. We successfully performed a revision bypass surgery using the remnant STA branch, resulting that the patient's motor weakness fully recovered again.
Double-barrel bypass is potentially more advantageous, especially for acute cerebral infarction in a large MCA territory, as compared to single-barrel bypass because diffusion-perfusion mismatch indicates a highly unstable state that needs urgent reperfusion therapy.2)3) In our experience, single-barrel bypass cannot improve the perfusion of both superior and inferior MCA divisions, and some patients present with cerebral infarction progression following single-barrel bypass surgery. Therefore, we have been recently performing double-barrel bypass in patients with large perfusion MCA territory defects. In this case, we think that the patient might have better outcome without recurrent cerebral infarction if he had initially underwent double-barrel bypass surgery.
Figures and Tables
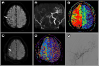 | Fig. 1(A) Initial diffusion-weighted imaging (DWI) revealed acute ischemic lesions (arrow) in the right hemisphere. (B) Magnetic resonance angiography revealed a right internal carotid artery occlusion. (C) Perfusion-weighted imaging (PWI) showed large perfusion defects in the right hemisphere. (D) Postoperative day 7 DWI revealed no interval changes in acute ischemic lesions (arrow). (E) Postoperative day 7 PWI demonstrated a markedly improved perfusion status in the right hemisphere. (F) Digital subtraction angiography (DSA) showed good parietal branch patency of the superficial temporal artery. |
 | Fig. 2(A) Diffusion-weighted imaging (DWI) showed recurrent acute ischemic lesions (arrows) in the right hemisphere six months after the initial bypass surgery. (B) Perfusion-weighted imaging (PWI) revealed large perfusion defects in the right hemisphere. (C) Digital subtraction angiography (DSA) demonstrated an occlusion of the superficial temporal artery (STA) bypass graft. (D) DSA revealed severe stenosis of the left proximal internal carotid artery (ICA). (E) After carotid artery stenting (CAS) of the left proximal ICA, DSA showed a slight blood flow increase into the right middle cerebral artery via the anterior communicating artery. (F) One day after left ICA CAS, DWI revealed an increase in acute ischemic lesions (arrows) in the right hemisphere. (G) PWI demonstrated a worsened perfusion status in the right hemisphere. |
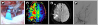 | Fig. 3(A) Intraoperative photography depicting the occluded bypass graft (arrow) and the STA frontal branch before end-to-side anastomosis to other M4 segments. (B) Postoperative day 7 PWI demonstrated an improved perfusion status in the right MCA territory. (C) Seven days after revision bypass surgery, the follow-up DWI revealed a slight increased ischemic lesions (arrows) in the right hemisphere. (D) Three months later, follow-up DSA, showed good STA frontal branch patency. |
References
1. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 03. 372(11):1009–1018.

2. Cherian J, Srinivasan V, Kan P, Duckworth EAM. Double-barrel superficial temporal artery-middle cerebral artery bypass: can it be considered “high-flow?”. Oper Neurosurg (Hagerstown). 2018; 03. 14(3):288–294.

3. Choi JH, Park HS. Emergent Double-barrel bypass shortly after intravenous administration of recombinant tissue plasminogen activator for acute ischemic stroke. J Cerebrovasc Endovasc Neurosurg. 2016; 09. 18(3):258–263.

4. Duckworth EA, Rao VY, Patel AJ. Double-barrel bypass for cerebral ischemia: technique, rationale, and preliminary experience with 10 consecutive cases. Neurosurgery. 2013; 09. 73:1 Suppl Operative. ons30–ons38. discussion ons37-8.

5. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 03. 372(11):1019–1030.
6. Halsey JH Jr, Morawetz RB, Blauenstein UW. The hemodynamic effect of STA-MCA bypass. Stroke. 1982; Mar-Apr. 13(2):163–167.

7. Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J Stroke. 2013; 01. 15(1):2–20.

8. Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011; 03. 68(3):723–729. discussion 729-30.

9. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 06. 372(24):2296–2306.

10. Kim MJ, Kang SY, Kwon SB, Jung S, Lee MJ, Jeong MG, et al. Contralateral cerebral infarction after unilateral carotid angioplasty with stenting. J Neurocrit Care. 2008; 12. 1(2):164–167.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download