This article has been
cited by other articles in ScienceCentral.
Abstract
Cavernous malformations (CMs) are angiographically occult vascular lesions, and their clinical presentations vary widely according to location of the lesion. Here, we reviewed three cases of CM located at the optic apparatus. All three patients experienced visual deterioration and underwent surgical resection. One achieved complete resection of the CM, whereas the others achieved subtotal resection. Visual symptoms of the two patients who achieved subtotal resection improved, but the visual symptom of the patient who achieved complete resection remained unchanged. One patient with subtotal resection presented postoperative improvement of visual symptoms but experienced deterioration in two years after surgical resection due to rebleeding from the remnant lesion, and he required a second operation. We recommend total resection of CM when feasible and regular follow-up after subtotal resection due to the risk of rebleeding.
Go to :

Keywords: Cavernous hemangioma, Optic nerve, Surgery, Hemorrhage
INTRODUCTION
Cavernous malformations (CMs) are vascular lesions comprising clusters of dilated capillary channels with thin walls devoid of intervening brain tissues.
7) The estimated incidence of CM is 0.4–0.8% and they can develop throughout the central nervous system.
4) Symptoms associated with CMs include headache, seizure and neurological deficits that are mostly determined by the location of the CMs. Cranial nerves are a rare site for CMs
12) with cranial nerve palsies as the most common presenting symptoms in these individuals.
9)12)
Given their locations, CMs at the optic apparatus are often mistaken for aneurysm, tumor bleeding or other brain pathologies, and the diagnosis remains challenging to healthcare professionals. Surgical intervention is also intricate and requires careful attention due to the delicate anatomy. Here, we present three cases of CM localized at the optic apparatus.
Go to :

CASE REPORT
Case 1
A 42-year-old woman presented with a 6-day history of severe headache and a 3-day history of blurred vision. She had visual field defect in the confrontation test with no other neurological deficits. Ophthalmologic evaluation revealed central scotoma on both eyes (
Fig. 1A) with normal visual acuity. Funduscopic examination showed intact optic discs with no abnormalities. A computed tomography scan showed a 1.3 cm high density ovoid lesion in the right suprasellar area (
Fig. 1B). Magnetic resonance (MR) imaging showed that the lesion was hypointense in the T2-weighed and T2
* gradient-echo (GRE) images and isointense in the T1-weighted images with no vascular abnormalities (
Fig. 1C–E). Transfemoral cerebral angiography did not reveal any vascular abnormalities. On the presumptive diagnosis of a hemorrhagic mass such as thrombosed aneurysm, meningioma or CM, craniotomy was performed via a right supraorbital keyhole approach to relieve the mass effect. Once the frontal lobes were retracted, a mulberry-shaped lesion was present at the junction of the right optic nerve and optic chiasm with no connection to the anterior cerebral artery (
Fig. 1F). The mass was completely removed with no damage to the optic apparatus Histologic analysis confirmed a diagnosis of CM (
Fig. 1G). The patient made an uneventful recovery after surgery. One year after surgery, the patient's visual symptoms remained unchanged.
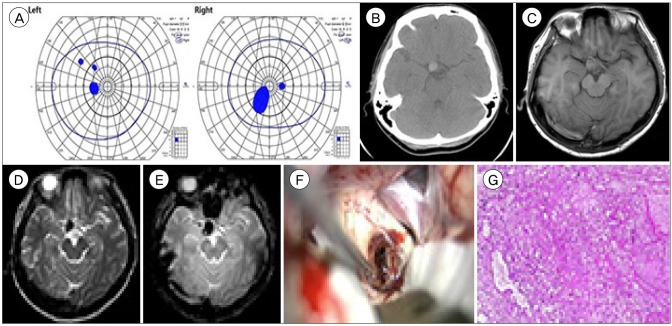 | Fig. 1Case 1. (A) Goldmann perimetry field test showing central scotoma on both eyes. (B) An axial computed tomography image showing the cavernous malformation located in the right suprasellar area. (C–E) Magnetic resonance images showing an approximately 1.5 cm-sized ovoid iso-intense lesion on T1-weighted images (C) and a hypointense lesion on T2-weighted (D) and T2* gradient-echo images (E) at the right suprasellar area. (F) Intra-operative photograph showing a CM embedded at the optic chiasm and optic nerve. (G) Pathological examination showing sinusoidal structures filled with hemorrhage, foamy macrophage infiltration, blood and fibrin clots (H&E stain, ×40).
|
Case 2
A 16-year-old boy presented progressive visual disturbance that started 5 months prior with headache. The headache had subsided; however, the visual symptoms persisted. His visual acuity was 0.02 in his left eye and 0.3 in his right eye. The Goldmann perimetry field test revealed a large central scotoma of his left eye field and a temporal hemianopsia of his right eye field (
Fig. 2A). Brain MR imaging showed a heterogeneous hemorrhagic mass in the optic chiasm, extending posteriorly along the bilateral optic tracts with no definitive contrast enhancement (
Fig. 2B–D). The presumptive diagnosis was an unusual CM involving the optic pathway. Following left pterional craniotomy and sylvian dissection, the optic chiasm, which was mottled with dark dots, was visualized. A small window was made on the lateral side of the optic chiasm. Chocolate-colored tissue mixed with old blood was partially evacuated (
Fig. 2E). The pathology report confirmed the diagnosis of CM. Following surgery, the patient's visual acuity in his left eye slightly improved (0.03/0.3). The central scotoma of his left eye field had resolved; however, he subsequently acquired a nasal hemianopsia in his left eye field. At the patient's 2-year follow-up, there were no episodes of headache, visual change, or documented bleeding on serial MR imaging (
Fig. 2F, G).
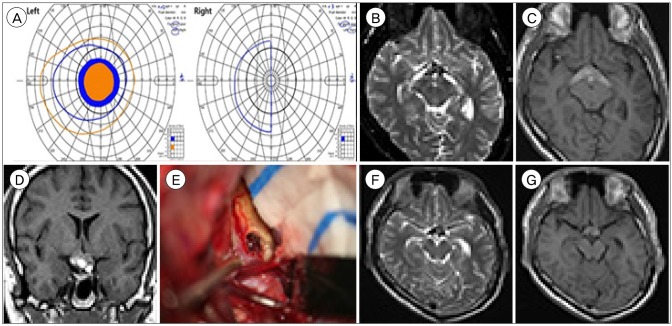 | Fig. 2Case 2. (A) Goldmann perimetry field test showing right temporal hemianopsia on the right eye and central scotoma on the left eye. (B–D) Magnetic resonance images showing inverted V-shaped hemorrhage at the optic chiasm and the bilateral optic tracts on axial T2-weighted (B) and non-enhanced T1-weighted images (C, D). Honeycomb appearance with multi-stage bleeding is observed at the optic chiasm. (E) Intraoperative photograph with chocolate-colored tissue mixed with old blood. (F, G) Postoperative T2-weighted (F) and T1-weighted (G) images showing residual mass at the optic chiasm.
|
Case 3
A 17-year-old boy complained of a 2-month history of visual field defects. Two months prior to this, he experienced sudden deterioration of visual acuity on his left eye and visual field defects on the right. His symptoms remained unchanged for the next 2 months. Upon admission, his visual acuity was 0.05 in the left eye and 0.7 in the right eye. Goldmann perimetry field test showed right homonymous hemianopsia without left eye foveal sparing. Brain MR imaging showed a poorly enhanced lesion 1.9 × 1.7 × 2.1 cm in size in the posteroinferior aspect of the optic chiasm (
Fig. 3A–C). The mass extended to the perimesencephalic cistern with multiple stages of hemorrhage. A differential diagnosis included CM, craniopharyngioma and germ cell tumor. To decompress the mass effect around the optic chiasm and improve the patient's visual symptoms, surgical decompression via subfrontal interhemispheric approach was performed. A brownish discoloration was noted around the left side of optic chiasm and tract. A dark chocolate-colored hematoma gushed out, and a mulberry-like lesion was removed. The pathological diagnosis was consistent with the CM. The postoperative MR imaging showed partial resection of CM (
Fig. 3D–F). At the patient's 1-year follow-up, his visual acuity improved (1.0/0.4), although visual field defect remained unchanged. However, 2 years after the first operation, the patient complained of worsening visual acuity (0.3/finger count 10 cm). Visual field examination showed impairments in which the left eye only detected the right lower quadrant in the visual field and the right eye had nasal hemianopsia. MR imaging revealed recurrent bleeding (
Fig. 3G–I). The patient underwent a second operation resulting in a partial resection. Immediately following the second operation, his visual acuity (0.4/finger count 10 cm) and field improved slightly.
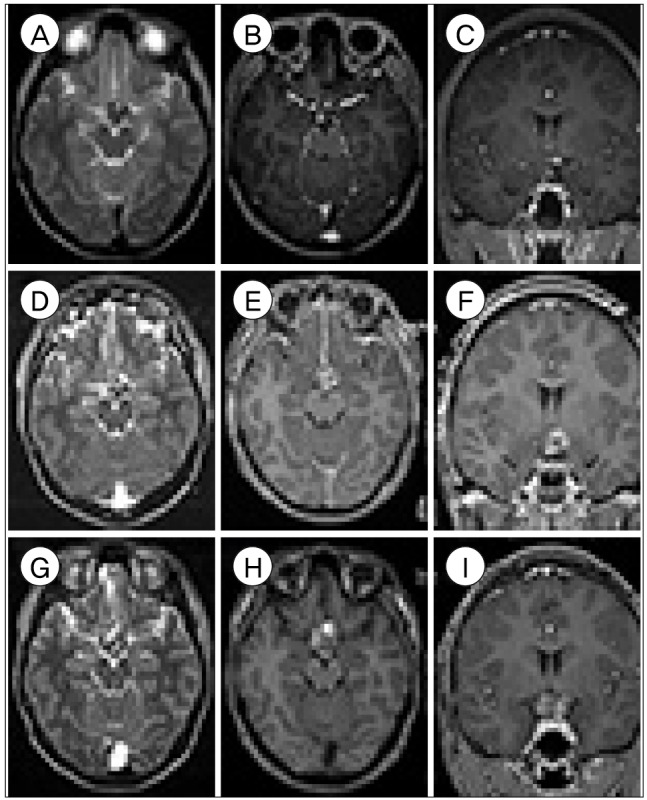 | Fig. 3Case 3. (A–C) Preoperative MR images showing a poorly enhanced lesion in the posteroinferior optic chiasm extending to the perimesencephalic cistern on T2-weighted (A) and enhanced T1-weighted (B, C) images. (D–F) Immediate postoperative MR image showing the partially resected lesion on T2-weighted (D) and enhanced T1-weighted (E, F). (G–I) Recurrent hemorrhage was identified on T2-weighted (G) and T1-weighted (H, I) MR images. MR = magnetic resonance.
|
Go to :

DISCUSSION
CMs are histologically benign lesions that can manifest with a wide variety of symptoms and signs, including incidental findings.
9) The key difference is the location of the lesion; the majority develops in the supratentorial area (80%) with the frontal and temporal lobes as the most frequent locations. The remainder develop in the infratentorial region (15%) and spinal cord (5%).
1)8) Interestingly, CMs affecting the cranial nerves are rare. According to a review by Rotondo et al.
9), the most commonly affected cranial nerve was the optic nerve, chiasm and tract (70%) followed by the facial/vestibulocochlear complex (12%). CMs involving the oculomotor nerve have been reported in eight patients, the trigeminal nerve in three patients, the trochlear nerve in two cases, and the abducens and hypoglossal nerves in one patient each.
2)9)10) Most cranial nerve CMs present with cranial nerve palsies of the affected cranial nerves. Direct nerve injury, compressive ischemia, edema, and/or irritation due to intralesional hemorrhage are thought to be the causes.
9)12)
T2
* GRE and susceptibility-weighted MR images are considered the most sensitive and specific imaging modalities for the diagnosis of CMs. Typically, the combination of a reticular core of mixed signal-intensity and a surrounding rim of decreased signal-intensity is indicative of CM.
5) However, a diagnostic uncertainty is not infrequent, and CMs can be confused with other lesions. In our cases, the CMs were all located around the optic chiasm, and given the diversity of pathology observed in this area, CM was not always the prime differential diagnosis. Because of their suprasellar location, CMs can be mistaken for craniopharyngioma, pituitary adenoma, meningioma, hemorrhagic metastasis and thrombosed aneurysm. To resolve this diagnostic uncertainty, appropriate investigations should be performed accordingly.
The natural history of CMs remains unclear, but the current consensus is that surgical excision is considered the standard practice for treating a symptomatic lesion. Recently, Tan and his colleagues reviewed 70 cases of CMs of the anterior visual pathway.
11) All the included patients presented visual symptoms of varying degrees. The majority underwent surgery; 53% achieved complete resection, 17% achieved subtotal resection and the rest had either hematoma evacuation or biopsy. Of those who achieved complete resection, 59% presented improved visual deficits. Among patients with subtotal resection, 50% presented improved visual deficits.
11)
In our cases, hemorrhage of CMs on the optic apparatus caused visual symptoms in all three patients. In the first patient, the total resection of the CM was achieved, whereas the last two patients achieved subtotal resection of the CM. The first two patients reported either slightly improved or unchanged visual symptoms on the follow-up visits. However, the last patient experienced deterioration in his symptoms, which required a second operation. To date, there is no literature available on the rebleeding rate of CMs after partial resection. However, based on our findings, we recommend gross total resection of CMs if possible. If subtotal resection is achieved, a second operation should be considered prior to rebleeding and/or clinical deterioration.
In addition to surgical management, non-surgical treatment of CMs, especially stereotactic radiosurgery, has been suggested and used at many centers. Studies have shown that radiosurgery reduced the hemorrhage risks in CMs,
3)6) but the majority of these studies were based on surgically inoperable lesions due to their locations. Moreover, radiosurgery has a high risk of deterioration of cranial nerve palsy because of the spatial proximity between targeted CM and cranial nerves.
Go to :

CONCLUSION
CMs at the cranial nerves are rare with the optic apparatus as the most commonly affected site among them. Bleeding from CMs can lead to cranial nerve palsy with a limited range of recovery. Complete resection is recommended but is not always feasible due to the delicate anatomy. If partial resection is achieved, a close follow-up, and repeat operation when possible, are recommended due to the risk of rebleeding.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download