Abstract
Purpose
Circulating patterns of predominant respiratory syncytial virus (RSV) genotypes in the community may be helpful in understanding molecular epidemiology and predicting future outbreaks of the RSV genotype. We investigated the association of genetic variations in RSV with acute severe bronchiolitis in infants.
Methods
We reviewed medical records of infants younger than 1 year of age hospitalized due to acute bronchiolitis between November 2016 and February 2017. Subjects were classified as severe or mild based on the use of mechanical or noninvasive ventilation. The associations between severity of the disease, sex, age at admission, oxygen saturation at admission and laboratory test results were analyzed. RSV sequence analysis was performed in the severe group.
Results
Among 114 infants, 80 underwent respiratory viral polymerase chain reaction using nasopharyngeal swab; of these, 53 (66.3%) showed positive for RSV. Of the 53 RSV-positive samples, 9 were categorized as the severe group and 44 were categorized as the mild group. Male sex, young age, longer duration of admission, minimum SaO2 at admission and bronchiolitis severity score were significantly correlated with disease severity in the severe group than in the mild group (all variables, P<0.001). Phylogenetic and sequence analysis in the severe group revealed 8 RSV-A, ON1 genotype and 1 RSV-B, BA4 genotype.
Conclusion
Phylogenetic types of RSV in subjects of the severe group were RSV-A, ON1 genotype or RSV-B, BA4 genotype which were prevalent in the Korean community at the same time. Our study showed that disease severity was not significantly associated with RSV genotypic evolution or antigenic drift in Korea during winter season 2016–17.
Go to : 
REFERENCES
1. Blount RE Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956; 92:544–9.
2. Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998; 79(Pt 9):2221–9.

3. Esposito S, Piralla A, Zampiero A, Bianchini S, Di Pietro G, Scala A, et al. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in Northern Italy in Five consecutive winter seasons. PLoS One. 2015; 10:e0129369.

4. Kim YJ, Kim DW, Lee WJ, Yun MR, Lee HY, Lee HS, et al. Rapid replacement of human respiratory syncytial virus A with the ON1 genotype having 72 nucleotide duplication in G gene. Infect Genet Evol. 2014; 26:103–12.

5. Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics. 2010; 125:342–9.

6. Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992; 145:106–9.

7. Øymar K, Skjerven HO, Mikalsen IB. Acute bronchiolitis in infants, a review. Scand J Trauma Resusc Emerg Med. 2014; 22:23.

8. da Silva LH, Spilki FR, Riccetto AG, de Almeida RS, Baracat EC, Arns CW. Genetic variability in the G protein gene of human respiratory syncytial virus isolated from the Campinas metropolitan region, Brazil. J Med Virol. 2008; 80:1653–60.
9. Yun MR, Kim AR, Lee HS, Kim DW, Lee WJ, Kim K, et al. Complete genome sequences of human respiratory syncytial virus genotype a and B isolates from South Korea. Genome Announc. 2015 Apr; 23:3. (2):pii: e00332-15.https://doi.org/10.1128/genomeA.00332-15.

10. Zhang H, Gao S, Lercher MJ, Hu S, Chen WH. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012; 40(Web Server issue):W569–72.

11. Anderson LJ, Hierholzer JC, Tsou C, Hendry RM, Fernie BF, Stone Y, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis. 1985; 151:626–33.

12. Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009; 459:1122–5.

13. Trento A, Galiano M, Videla C, Carballal G, García-Barreno B, Melero JA, et al. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J Gen Virol. 2003; 84(Pt 11):3115–20.

14. Trento A, Casas I, Calderón A, Garcia-Garcia ML, Calvo C, Perez-Breña P, et al. Ten years of global evolution of the human respiratory syncytial virus BA genotype with a 60-nucleotide duplication in the G protein gene. J Virol. 2010; 84:7500–12.

15. Hwang SJ, Park DJ, Gu PT, Koo HS, Lee MO. Characteristics of respiratory syncytial virus isolated from acute respiratory infectious disease in Busan. J Bacteriol Virol. 2016; 46:173–80.

16. Baek YH, Choi EH, Song MS, Pascua PN, Kwon HI, Park SJ, et al. Prevalence and genetic characterization of respiratory syncytial virus (RSV) in hospitalized children in Korea. Arch Virol. 2012; 157:1039–50.

17. Eshaghi A, Duvvuri VR, Lai R, Nadarajah JT, Li A, Patel SN, et al. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: a novel genotype with a 72 nucleotide G gene duplication. PLoS One. 2012; 7:e32807.

18. Johansson C. Respiratory syncytial virus infection: an innate perspective. F1000Res. 2016; 5:2898.

19. Jeong Y, Hwang JH, Kwon JY, Shin J, Kwon JH, Han K, et al. Prediction of the severity and length of hospital stay in infants with acute bronchiolitis using the severity score. Allergy Asthma Respir Dis. 2016; 4:429–35.

20. Cha MA, Woo YR, Kim HJ, Kim MS, Ahn YH. Factors associated with obesity of acute bronchiolitis in infants: association of obesity with disease severity. Allergy Asthma Respir Dis. 2015; 3:281–7.

21. Yoshihara K, Le MN, Okamoto M, Wadagni AC, Nguyen HA, Toizumi M, et al. Association of RSV-A ON1 genotype with increased pediatric acute lower respiratory tract infection in Vietnam. Sci Rep. 2016; 6:27856.

22. Panayiotou C, Richter J, Koliou M, Kalogirou N, Georgiou E, Christo-doulou C. Epidemiology of respiratory syncytial virus in children in Cy-prus during three consecutive winter seasons (2010-2013): age distribution, seasonality and association between prevalent genotypes and disease severity. Epidemiol Infect. 2014; 142:2406–11.

Go to : 
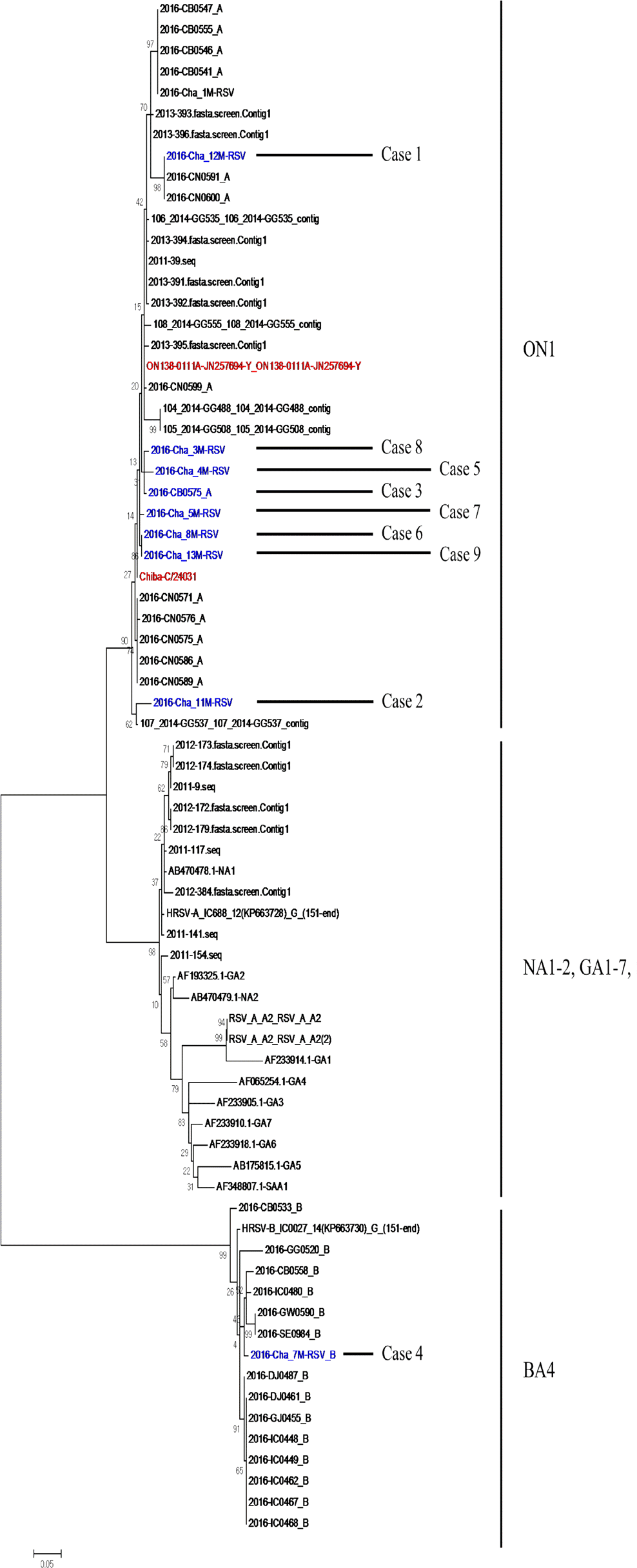 | Fig. 1.Phylogenetic analysis of the RSV-A/RSV-B strains using partial G protein sequences and reference sequences of identified genotypes. All cases are classified into RSV-A, ON1 genotype (n=8) and RSV-B, BA4 genotype (n=1) that are currently circulatin. RSV, respiratory syncytial virus. |
Table 1.
Demographic characteristics of the study subjects
| Characteristic |
Group |
P-value | |
|---|---|---|---|
| Mild (n=44) | Severe (n=9) | ||
| Age (mo) | 2.5 (0.5–4.8) | 1.0 (1.0–2.0) | 0.011∗ |
| Length of admission (day) | 3.0 (2.3–4.0) | 6.0 (4.0–10.0) | <0.001∗ |
| Male sex, n (%) | 23 (52) | 9 (100) | <0.001∗ |
| Minimum SaO2 at admission (%) | 98.0 (97.0–99.0) | 83.0 (80.0–92.5) | <0.001∗ |
| BSS | 6.0 (5.0–7.0) | 13.0 (11.0–14.0) | <0.001∗ |
| VBGA pH | 7.37 (7.35–7.42) | 7.28 (7.13–7.32) | <0.001∗ |
| VBGA pCO2 (mmHg) | 40.8 (35.1–45.6) | 62.3 (54.6–95.0) | <0.001∗ |
| VBGA HCO3 (mmol/L) | 23.5 (20.9–24.8) | 28.2 (25.5–31.2) | <0.001∗ |
| WBCs at admission (×103/mm3) | 11.0 (9.09–13.2) | 8.1 (7.79–10.1) | 0.086 |
| Hemoglobin at admission (g/dL) | 11.7 (10.9–12.3) | 10.8 (9.8–12.0) | 0.128 |
| C-reactive protein at admission (mg/dL) | 0.4 (0.0–0.97) | 0.3 (0.13–0.52) | 0.935 |
Table 2.
Clinical characteristics of the study subjects in the severe group
| Case | Sex | Age (day) | Length of admission (day) | Minimum SaO2 at admission (%) | BSS | Initial VBGA (pH–pCO2∗–HCO3#) | Ventilator mode | RSV (type/genotype) | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 24 | 9 | 94 | 12 | 7.17–97.4–34.9 | Invasive | A/ON1 | None |
| 2 | Male | 39 | 12 | 8 | 16 | 6.73–164.8–21.1 | Invasive | A/ON1 | Mild hypoxic encephalopathy |
| 3 | Male | 44 | 6 | 70 | 13 | 7.08–92.6–26.9 | Invasive | A/ON1 | None |
| 4 | Male | 47 | 11 | 91 | 11 | 7.23–80.1–32.9 | Invasive | B/BA4 | None |
| 5 | Male | 54 | 6 | 93 | 14 | 7.31–59.5–29.4 | HFNC | A/ON1 | None |
| 6 | Male | 59 | 4 | 92 | 7 | 7.32–51.6–26.5 | HFNC | A/ON1 | None |
| 7 | Male | 70 | 6 | 90 | 14 | 7.31–57.6–28.5 | HFNC | A/ON1 | None |
| 8 | Male | 71 | 6 | 90 | 11 | 7.28–62.3–28.2 | nCPAP | A/ON1 | None |
| 9 | Male | 87 | 4 | 90 | 13 | 7.36–44.4–24.5 | HFNC | A/ON1 | None |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download