Abstract
Background
Atopic dermatitis (AD) is a chronic or chronically relapsing, eczematous, severely pruritic inflammatory skin disorder. Korean red ginseng (KRG) has been shown previously to exhibit diverse biological effects including anti-inflammatory and antipruritic effects in a murine model.
Objective
We aimed to investigate the beneficial effects of KRG on AD patients, to determine whether there was improvement in disease severity, skin barrier function, pruritus and sleep disturbance relief.
Methods
An open, noncomparative clinical study that utilized KRG tablets (500 mg/tablet) was conducted. This study included 41 patients with mild to moderate AD diagnosed by the Korean atopic dermatitis guidelines. Three visits to the hospital at days 1, 28±7, and 56±7 for evaluation were made. The effects of KRG were assessed by measuring eczema area and severity index (EASI) score, transepidermal water loss (TEWL), the visual analogue scale (VAS), total amount of topical agents used in recent 8 weeks and investigator global assessment (IGA).
Results
Patients taking KRG tablets showed significant decreases in EASI score and TEWL, and the VAS of pruritus and sleep disturbance were significantly reduced. The amount of topical agents used was reduced but not by a statistically significant amount. IGA at the third visit showed improvement of AD compared to the second visit, but the difference was not statistically significant.
Atopic dermatitis (AD) is a chronic or chronically relapsing, eczematous, severely pruritic skin disorder1. It is mostly associated with immunoglobulin E (IgE) elevation and skin barrier dysfunction due to decreased filaggrin expression1. AD correlates with the expression of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1 from keratinocytes, mast cells and dendritic cells. Acute AD is associated with the production of T helper type 2 (Th2) cytokines, IL-4 and IL-13. Langerhans cells (LCs) appear to play an important role in cutaneous allergen presentation to Th2 cells. During the chronic phase of AD, there is a switch to Th1-like cells which primarily produce interferon (IFN)-γ. Other inflammatory mediators, such as histamine, protease, neuropeptides and IL-13, are also released and intensify pruritus which leads to scratching and impairment of skin barrier function2. Recently, T regulatory (Treg) cells, a subtype of T cells, have been reported to play an essential role in immune tolerance and are characterized by the dominant transcription of FOXP3, a transcription factor of the forkhead/winged helix family3. Treg cells suppress immune responses by interacting with effector T cells or antigen-presenting cells. Recent clinical research has found that parents, particularly mothers, who have a history of atopic disease could have babies with less stable FOXP3+ Treg cells in cord blood. These babies have the onset of AD less than 1 year after birth. So that, it is likely that the abnormal numbers of Treg cells weaken the inhibition of TH2 lymphocytes, thus resulting in AD inflammation4.
For the management of AD, early treatment at the onset of symptoms and the prevention of recurrence are important. Use of topical steroid is essential for successful management; however, there are controversies regarding its long-term use. Topical calcineurin inhibitor (TCI) was initially developed as nonsteroidal immunomodulator. However, burning sensations were frequently observed after TCI treatment. Antihistamine treatment is insufficient to inhibit pruritus in AD patients. Therefore, it is necessary to develop an ideal therapy of AD that takes into account side effects as well as benefits.
Korean red ginseng (KRG, the steamed root of Panax ginseng C.A. Meyer, family Araliaceae) is frequently taken orally as a remedy in United States and Europe as well as Asian countries. The major active ingredients of ginseng are the triterpene glycosides also known as ginsenosides, which contain an aglycone with a dammarane skeleton5. Pure ginsenosides have been reported to promote anti-allergic and anti-inflammatory effects67. Recently, we have demonstrated that oral administration of KRG could inhibit the inflammation of AD-like skin lesions in NC/Nga mice by suppressing LCs, Th1 related cytokines, and Treg cells8. In another study, we demonstrated that oral administration of KRG could inhibit the development of the AD-like skin lesions in NC/Nga mice both systemically and in lesional skin by suppressing TSLP, DCs, and in part, the Th2 response. Taken together, it is suggested that oral administration of KRG may be a novel approach to the prevention and treatment of early lesions in AD9.
Thus far however, there has been no evaluation of KRG on human AD patients. Therefore, in the present clinical trial, we aimed to investigate the potentially beneficial effects of KRG, with regard to improvement in disease severity, skin barrier function, and pruritus and sleep disturbance relief.
For this study, 41 patients with mild to moderate AD who visited The Catholic University of Korea, Incheon St. Mary's Hospital (Incheon, Korea) and Hallym University Kangnam Sacred Heart Hospital (Seoul, Korea) between February 2016 and January 2017 were included (CMC IRB: OC16HIMI0005). The inclusion criteria were defined as follows: 1) age 4 years or older, 2) eczema area and severity index (EASI) score 5 or higher, 3) patients who voluntarily agreed to participate in the study (in case of patients under age 18, a legal guardian agreed to allow them to participate). Patients who were allergic to ginseng were excluded.
An open, noncomparative clinical study that utilized KRG tablets (500 mg/tablet) was conducted. This study included 41 patients with mild to moderate AD diagnosed by the criteria of the Korean AD guidelines. Three visits to the hospital were made with day 1 used for baseline disease evaluation. Patients revisited at day 28±7, and day 56±7 for further evaluation. At all three visits, EASI score was estimated, and transepidermal water loss (TEWL) was measured using a Tewameter TM 210 (Courage-Khazaha, Koln, Germany) from below both cubital fossae. Pruritus relief and sleep disturbance were assessed with a visual analogue scale (VAS) by the patients themselves. This scale assigns a numeric value to pruritus severity from 1 to 10, with 10 being most severe. Medication history of topical agents during the past two months was also recorded. The total amounts used were calculated. From the day of the first visit, patients who were over the age of 17 were instructed to take 1 g of ginseng tablets twice a day (2 g/d) orally, and patients between 4 and 17 were instructed to consume 0.5 g of ginseng extractions twice a day (1 g/d) for 8 weeks. On the second and third visit, all the parameters were evaluated again. The EASI score was calculated for the affected area and eczema severity by a primary investigator. TEWL was measured on the same spot of both forearms of the patient regardless of the presence of eczema lesion, according to the manufacturer's guidelines. From the second visit, investigator global assessment (IGA), which is defined as an overall evaluation of AD severity using an ordinal scale or an ordered categorical variable that was scored using points by an investigator or a physician, was checked based on a comparison of the improvement from the last visit. Adverse drug reactions were evaluated in all patients during the second and third visits. Drugs used by patients for the treatment of AD were accepted and no new drugs were prescribed. However, adjustment of dosage according to symptoms was allowed.
Statistical analyses were performed using the Wilcoxon signed-rank test for significant differences in the EASI score, TEWL, VAS scores for pruritus and sleep disturbance, and total amount of topical agent, after taking KRG. The p-values <0.05 were regarded as statistically significant. Data were analyzed using R statistics ver. 3.2 (R Foundation, Vienna, Austria).
Of the 41 patients who were initially enrolled, 6 were excluded from the statistical analysis for not visiting the hospital at least once after administration of KRG tablet. The remaining 35 individuals who were evaluated at least once in addition to the baseline evaluation were included in the statistical analysis. Compared to the data at first visit, disease severity was significantly improved after 8 weeks. No statistically significant difference was noted on the quantities of topical agents used.
EASI score was analyzed statistically using the Wilcoxon signed-rank test. EASI scores during the study period are shown in Fig. 1. The mean EASI score was 11.13 at baseline and this had diminished to 9.21 by visit 3. The EASI score was significantly lower after eight weeks of oral KRG (p=0.026).
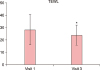
TEWL results were interpreted in the same way as for the EASI score analysis. The changes in TEWL values are shown in Fig. 2. TEWL values as measured on both forearms of all patients changed from 28.32 at baseline to 23.78 (p=0.040) when measured on visit 3. It can be said that TEWL was statistically significantly higher before the intake of KRG than after the intake of KRG.
Stastical analysis of the results of VAS scores for itching and sleep disturbance was conducted. The changes in VAS scores are shown in Fig. 3. Both were significantly decreased in patients at visit 3. From 5.23 (pruritus) and 3.37 (sleep disturbance) at baseline, decreases of −1.63 (p=0.0002) and −1.4 (p=0.0001) at visit 3 were observed respectively.
The amount of topical agents used showed a similar pattern with the severity of the disease. From 67.7 g at baseline, decreases of −13.2 g at visit 3 were observed. However, this change was not significant (p=0.2365).
The prevalence of AD has increased gradually in many countries. In Korea, from 1995 to 2008, the prevalence of AD in children aged 6 to 12 years old increased from 16.3% to 19.1% and AD prevalence in children aged 12 to 15 years old increased from 7.3% to 9.2%. The causes are presumed to result from increasing exposure to antigens derived from environmental pollution. These include nitrogenous compounds and fine dusts emissions from vehicles, pollutants from industrial sites and volatile organic compounds, changes in residential environments and possibly Westernization of lifestyle. AD is becoming an increasingly burdensome public health problem with large socioeconomic costs. These costs include not only those of clinical treatment but also absence from school and work, decreased ability to perform tasks, loss of jobs and unemployment, decreased incomes, increased medical expenses, and lower quality of life due to the restriction of social activities.
AD is a chronic, relapsing inflammatory skin disease usually commencing in childhood, so not only are medical treatments for prevention of recurrence and maintenance important, but also doctor-patient relationships. Prevalence of AD is highest in children less than two years of age and diminishes with increasing age. The most common outcome of AD is for it to resolve during childhood, but it may persist into adult life or recur in the teenage or early adult years. Because of these characteristics, the decisions about treatment are often the responsibility of parents.
Systemic therapies of AD including glucocorticoids and cyclosporines are generally either directed at suppressing the underlying inflammation or targeting peripheral or central itch pathways. However, because of concern about side effects of drugs used in children, the uses of systemic agents are limited in childhood AD. Topical steroid and TCI are often used as a first-line therapy in AD because they usually have a lower risk of adverse effects than the systemic medications. However, many parents of AD patients are so concerned about these medications they refuse to use either topical or systemic steroids. TCI is safer than topical steroids, but less effective and has more local side effects like burning, itching sensation and erythema10. Therefore, effective and safe adjuvant treatments of AD are needed. Results from several studies show that patients with AD may benefit from the comprehensive therapy as well as a supplemental therapy such as evening primrose11. KRG is frequently taken orally as a remedy, especially in Asian countries. KRG in particular is also known to have anti-allergic effects61213.
Previously, we have shown that KRG therapy may be beneficial for the treatment for AD using the NC/Nga murine AD model. Systemic KRG extract significantly reduced the total clinical severity score, ear thickness and the level of serum IgE in the AD mouse model. KRG not only decreased TNF-α, IFN-γ and substance P but also reduced the infiltration of FOXP3+ regulatory T (Treg) cells and CD1a+ LCs in the lesions. These results suggest that KRG extract may be a potential therapeutic modality for AD8.
In a subsequent study we further investigated the effects and mechanisms of KRG on the prevention and treatment of early lesions of AD. KRG significantly reduced ear thickness and significantly prevented the increase in TEWL induced by TNCB. The serum IgE level was significantly lower in the KRG group. Orally administrated KRG may inhibit the development of AD-like skin lesions in NC/Nga mice by modifying TSLP, DCs, and at least in part, the Th2 response9.
More recently, we investigated whether the therapeutic effect of KRG on AD in mice is mediated by reducing the pruritus, or by suppressing the inflammation. KRG decreased clinical signs and symptoms and the number of scratching movements. The mechanism of these effects may be a reduction in mast cell infiltration and/or suppression of systemic IL-31 and IgE. Second, KRG protected skin barrier function as indicated by TEWL elevation. Third, KRG exerts anti-inflammatory effects by reducing the mRNA levels of TNF-α and by histopathologic findings such as markedly reducing the extent of epidermal hyperplasia, hyperkeratosis, parakeratosis, and dermal infiltration of leukocytes and mast cells. Thus, proactive systemic administration of KRG may effectively reduce early allergic sensitization and suppress the Th2 response. Moreover, restricting scratching behavior suppresses the vicious cycle of itching and scratching, thus reducing clinical and systemic inflammation14.
In the present study, KRG showed clinical improvement in human AD patients in similar fashion to the murine model. The results showed significant reductions in the EASI score, TEWL, degree of pruritus and sleep disturbance after 8 weeks of taking KRG.
TEWL is linked to damage of the stratum corneum lipid barrier and a subsequent loss of corneocyte adhesion. Skin barrier abnormalities, including increased TEWL and decreased skin hydration, are biomarkers for the severity and itch intensity in AD15. It is noteworthy that KRG not only improves the general well-being of the patient, but also restores skin barrier function through reduction of TEWL.
Subjective pruritus and sleep disturbance decreased in the human model just as the scratching behavior improved in the murine model. Restriction of scratching in the early stages reduces clinical and systemic inflammation by preventing the vicious itching-scratching cycle, thus reducing clinical and systemic inflammation.
This is the first study to demonstrate that supplementary administration of KRG on human AD patients improves clinical symptom such as pruritus and sleep disturbances, as well as objective findings as noted in TEWL and EASI scores. The results of this study suggest that KRG can be safely used as a supplement remedy leading to clinical improvement of AD, can improve overall quality of life, and has potential for further development.
Limitations of present study it that we cannot fully exclude effects of other drugs used by patients for the treatment of AD, despite there was no significant difference in dosage between before and after the experiment. Additionally, this study has a relatively small number of subjects and is non-controlled and short-term study.
Thus further large-scale, well controlled clinical trials will likely confirm the usefulness of KRG as an adjunctive therapy for AD patients, and the effects of KRG are certainly worth investigating for therapeutic efficacy on other inflammatory skin diseases.
Figures and Tables
References
2. Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006; 118:178–189.

3. Szegedi A, Baráth S, Nagy G, Szodoray P, Gál M, Sipka S, et al. Regulatory T cells in atopic dermatitis: epidermal dendritic cell clusters may contribute to their local expansion. Br J Dermatol. 2009; 160:984–993.

4. Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012; 67:380–389.

5. Shibata S, Fujita M, Itokawa H, Tanaka O, Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo). 1963; 11:759–761.

6. Bae EA, Trinh HT, Yoon HK, Kim DH. Compound K, a metabolite of ginsenoside Rb1, inhibits passive cutaneous anaphylaxis reaction in mice. J Ginseng Res. 2009; 33:93–98.

7. Ro JY, Ahn YS, Kim KH. Inhibitory effect of ginsenoside on the mediator release in the guinea pig lung mast cells activated by specific antigen-antibody reactions. Int J Immunopharmacol. 1998; 20:625–641.

8. Lee JH, Cho SH. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011; 133:810–817.

9. Cho E, Cho SH. Effects of Korean red ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013; 145:294–302.

10. Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011; 30:71–86.

12. Matsuda H, Samukawa K, Kubo M. Anti-inflammatory activity of ginsenoside ro. Planta Med. 1990; 56:19–23.
13. Park EK, Choo MK, Kim EJ, Han MJ, Kim DH. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003; 26:1581–1584.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download