Abstract
Background
Tumors with cysts often correlate with gliomas, metastatic tumors, or hemangioblastomas, which require differentiation.
Methods
Thirty-eight cases of cyst associated-meningioma based on preoperative radiologic studies and histologic confirmations were reviewed from November 1998 to July 2017.
Results
A total of 395 cases of meningioma were observed in the 20 years, and surgical treatment of intracranial meningioma was performed in 120 cases. Thirty-eight (9.6%) cases of cyst associated meningiomas were analyzed. Nauta type I was the most common type of cyst (39.5%) and the most frequent histopathological subtype was meningothelial type (36.8%).
Conclusion
Statistically there were no significant associations between meningioma histopathological type and associated cysts; however, the rate of World Health Organization grade II was higher in cyst associated meningiomas than in unrelated meningiomas. This correlation was weak, in accordance with the meningioma grade.
Meningioma is commonly a solid-form tumor, and intracranial tumors associated with the cyst appears frequently in gliomas and metastatic tumors [1]. The incidences of meningiomas that are associated with cystic lesions are rare. According to the studies on cystic meningioma, this disease has a recorded frequency of 1.6–11.7% [1345678]. During a 35-year period, Fortuna et al. [1] reported 22 cystic meningiomas among 1,313 intracranial meningiomas (1.7%).
Four different types of classification, which have been suggested by Nauta et al. [2] are most widely used (Table 1). The histopathological classification and Simpson grade is meaningful for predicting prognosis of meningioma. The correlation between an associated cyst and the histopathologic type of meningioma are not clear but if the factors are interrelated, each could help predict the prognosis for this disease. In the current study, we analyzed the association between Nauta classification type, histopathologic subtype and World Health Organization (WHO) grade in meningiomas with cysts.
Several previous documents have formulated hypotheses for how cysts form in meningiomas. According the degenerative phenomenon hypothesis, the development of the cavity is due to intracellular regressive processes that cause macrocavitation, such as vacuolar, myxomatous, mucoid and fatty degeneration [910]. Russell et al. [11] argued that arteriolar hyalinization in the necrotic tissue of tumors that develop into the intratumoral cavity, or “oasis phenomenon” validates the ischemic origin of the degenerative phenomena. It has also been reported that the expansion of cysts that are bigger than the tumor sometimes triggers mass effect and clinical deterioration [4]. The feasibility of intratumoral cysts originating from previous intratumoral hemorrhage in angioblastic meningioma has been suggested by several authors [1012131415]. In case of peripheral cyst, a few authors have pointed out that Nauta classification type III is made by reactive gliosis against meningioma [51316]. Rengachary et al. [5] claimed that this hypothesis is verified by the positivity for glial fibrillary acid protein, which can be determined through an immunoperoxidase assay of the cyst wall. It has also been suggested that the peritumoral cyst of Nauta type IV is caused by extension of the cerebrospinal fluid (CSF) into the subarachnoid space, compressed by the tumor [171819]. Further, cases of tumor communication with the subarachinoid space through pneumocephalogram have also been reported [18].
Cystic meningioma are diagnosed based on radiologic findings and classified into four subtypes, as suggested by Nauta et al. [2] (Table 1). Associated cysts are categorized based on the relation between the tumor and the brain and of the 166 cases reported by Fortuna et al. [1], type I was the most frequent (32%).
Macroscopically, type I cysts reside in the middle of the tumor and are almost completely surrounded by tumor (Fig. 1). Type II cyst are located at the tumor periphery and in type II cysts, the margin of the tumor and the rim of tumor cell are attenuated along the margin of the cyst (Fig. 2). Type III cysts are not located inside the tumor but rather adjacent the brain (Fig. 3) and some cases assume the form of a cyst nest. Type IV cysts shows the form of CSF, which is localized to the subarachnoid space between the tumor and the brain exterior (Fig. 4). Sridhar et al. [20] classified 17 cases of cystic meningioma into peritumoral and intramural cysts, and intratumoral cysts that fell under Nauta type I and II were most common (11 cases, 68.8%). Additionally, the study of Liu et al. [21] found 14 intratumoral cysts among 21 cases (66.7%).
Cystic lesions within the intracranial neoplasm are usually associated with gliomas, hemangioblastomas, or metastatic tumors. Cyst-associated meningiomas are radiologically confusing cyst-associated tumors; and while computed tomography (CT) records allow 90% accuracy for diagnosing intracranial meningiomas [222324]. Fortuna et al. [1] has revealed that CTs barely allow 38% accuracy when the meninigioma is associated with a cyst, although magnetic resonance imaging (MRI) (80%) allows higher diagnostic accuracy than CT (50%) [7].
There are several radiologic findings that can differentiate between cystic meningioma and other cyst-associated tumors. Meningiomas display highly enhanced mass, while lower grade gliomas do not, and meningiomas attach to the dura and can be identified by a characteristic dural tail sign on T1-weighted image (T1WI) contrast enhancement MRI. Furthermore, meningiomas are located extra-axially while other cyst-associated tumors are not. About 95% of hemangioblastomas are located in the posterior fossa and rarely extend beyond the cerebellum into the cerebellopontine angle [25]. In typical meningioma, peritumoral edema is not prominent; however, cyst-associated meningiomas show peritumoral edema in variable ranges that do not provide differential radiologic findings.
This study includes 123 cases of surgical treatments from the 395 cases of radiologically-diagnosed meningioma from November 1998 to July 2017. All spinal meningiomas were excluded from the current series. Of the 123 cases, two cases that did not have a confirmed histopathological subtype in their medical charts, one case of skull meningioma, and total 120 cases of intracranial meningioma were analyzed. Based on radiological finding, there were 38 cases of meningioma correlated with cysts. In the current series, cystic meningiomas were classified using Nauta's classification [2]. All 120 cases of surgically-removed meningiomas were verified by a pathologist for histopathological subtype and WHO grade. Meningioma cysts were confirmed through MRI or CT. The subtype of cystic meningioma was categorized according to classification suggested by Nauta et al. [2], and patient age, gender, and tumor location were analyzed in this research. Also, statistical analysis was performed using SPSS version 21 (IBM Corp., Armonk, NY, USA). The cohort characteristics of cystic and non-cystic meningiomas were analyzed by t-tests. Fisher's exact test was used to analyze the relationship between tumor location and histopathologic subtype and WHO grade. Lastly, p-values <0.05 were designated as significant, and this study was approved by the Institutional Review Board (IRB No. GNUH 2017-09-028-001).
Of the 395 meningiomas diagnosed from November 1998 to July 2017, 38 cases were associated with cysts and accounted for 9.6% of the intracranial primary meningiomas. The analyzed data are summarized in Table 2. The mean age of patients with cystic meningiomas was 55.2 years (±9.4 standard deviation; range, 34–74), cystic meningiomas were more frequent in women (24 vs. 14 in male), and tumors were most common in the cerebral convexity. The most common histopathologic subtype was meningothelial type (36.8%), and most cases were WHO grade I (78.9%). Cases are summarized in Table 3 according to Nauta classification types.
The solid mass portion represented strong but variable enhancement (homogenous or heterogenous) on contrast-enhanced T1WI in MRI scan, and on T2-weighted image (T2WI) showed variable signal, from isosignal to low signal intensities. The cystic portion commonly showed high signal intensity at T2WI.
In the current series, Nauta classification type I was most frequent (39.5%) and type III was least observed (7.9%). There were no significant differences in ages (p=0.120) between patients with or without cysts. Fortuna et al. [1] described that the most common type of cystic meningioma is type III, and type IV is most frequent in Sridhar's series [20]. However, in the cumulative results of previous series, type I was the most frequent (Table 3). Some authors have classified the cystic portion into intratumoral and peritumoral types [42627], and according to this classification, peritumoral type cysts were more frequent (45 vs. 30 intratumoral type).
There were three cases of tumor recurrence, and in all cases, tumors were located within the cerebral convexities and showed histopathologic progression (Table 4). Only one video record of the last case with recurrence was available, and it is important to determine if the accompanied cysts were removed by reviewing the operation record. Our records revealed that there was one case with clear removal of the associated cyst in which the cyst was Nauta type I. Because the cyst was intratumoral type and the tumor and cyst were removed at the same time with Simpson grade II. The other tumors were removed with Simpson grade III and IV however in these cases, it is not clear if the cyst walls were removed. Since these cases lacked information about the cyst wall in the operation records, we concluded that the cyst walls were not identified and removed. Therefore, it is unclear whether the removal of the cyst wall increases the risk of recurrence. However, histopathological subtype is atypical meningioma (WHO grade II), and in two out of three cases it was removed by Simpson grade III and IV, and this seems to be a risk factor for recurrence. In our study, the most common histopathological type was meningothelial type (WHO grade I), and the rate of meningioma accompanied by cyst was higher in WHO grade II than in WHO grade I. For initial meningioma diagnoses, WHO grade III was only observed in one out of 118 cases, and this difference is of minor statistical significance.
In this investigation, all studies that took MRI and CT scans as diagnostic modalities when cysts were suspected inside the tumor or outside but adjacent to the tumor were reviewed. Among 38 meningiomas with cysts, 27 cases used both MRI and CT, while 10 cases used only MRI and one applied solely CT. When using MRI, it was easier to check the cyst portion of low signal intensity in the contrast-enhanced T1WI. When a low signal intensity was unclearly observed in T2WI and the tumor showed cystic morphology, it was possible to define whether or not a cyst was present through using CT and checking calcification. Hence, CT images provided a meaningful diagnostic tool when T2WI signal intensity was vague in lesions suspected to be small cysts.
The incidence of cystic meningioma varies among previous studies. Many authors described that 1.6–10% of all intracranial meningiomas are associated with cystic lesions [13456720], and up to 10% of meningiomas in children are associated with cysts [122829]. Fortuna et al. [1] reported cysts in 22 (1.7%) of 1,313 intracranial meningioma cases, and Sridhar et al. [20] observed cysts in 17 cases (7.3%) of cystic meningiomas in their series between 1984 and 1993. In our current series, there were 38 cases (9.6%) of cyst associated meningiomas out of 395 meningioma cases.
If we assume that the pathogenesis of cystic lesions is caused by degenerative phenomenon in type I and type II cysts, which are the most common subtypes, the longer duration of disease likely to increase the development of a cystic portion. There may be a variety of medical and non-medical factors that increase the interval from when the patient first complains of symptoms to proper diagnosis. Therefore, the basal skull or meningioma of the posterior fossa, which are likely to have relatively rapid symptoms such as mass effect or non-communicating hydrocephalus, will not have enough time to progress to cystic degeneration. In this study, tumor-associated cystic lesions at this location were observed in 1.2–8.5% of entire cystic meningiomas. Non-medical factors, such as patient subjective symptoms and need for treatment, socioeconomic approaches to medical care, and medical policy implications for using diagnostic imaging tools may cause statistical differences in the interval from the initial symptom presentation to diagnosis.
Nauta categorized cysts into four types based on the relationships with the tumor, the associated cysts, and the surrounding brain, and the features of each type are described above. The relationship between the presence of cysts and surrounding structures was relatively well identified in T2WI, and enhancement of the cystic wall could be identified in contrast enhanced T1WI.
Diffusion-weighted images (DWI) and apparent diffusion coefficient (ADC) maps also provide diagnostic information for patients with intracranial lesions, such as cellularity, but the relationship between ADC and histopathology is not clear. Kono et al. [30] and Filippi et al. [31] suggested correlations between ADC and histopathological meningioma subtypes using DWI and ADC maps to estimate relative tumor cellularity. Filippi et al. [31] reported that ADC values were lower than normal brain values and were hyperintense on DWI and hypointense on ADC maps. Sanverdi et al. [32] also found no additional value of ADC coefficients in predicting the grade of meningiomas, but Kono et al. [30] analyzed 18 meningiomas and found no significant relationships between tumor ADC and histologic classification. The Chen et al. [33] reported that meningioma cystic portion were hypointense or mildly hyperintense on DWI, and the ADC ratios for the cystic parts of two type I cystic meningiomas were 1.25 and 0.82. Furthermore, Surov et al. [34] analyzed a series of 389 patients and found that grade I meningiomas have significantly higher ADC values compared to WHO grade II and III.
Chen et al. [33] reported that ADC values can vary according to the contents of cystic lesions that the increase in fluid content, which results in hypointense DWI signal intensity because the free movement of water molecules is less restricted. In addition, Nauta type I cysts, which are thought to be caused by tumor necrosis, have mildly hyperintense signal intensities because they contain residual cells that restrict the movement of free water [33]. There were studies that grade I meningiomas have significantly higher ADC values than WHO grade II and III [3435], but this observation needs further clarification to better understand the correlation between cyst type and DWI signal intensity.
In our study, the most common histopathological type was meningothelial type (WHO grade I). These results are similar those of some other authors [12735], but fibrous [36] and atypical [33] meningiomas are also frequent in other series. Studies that have tried to analyze the correlation between radiologic findings and pathological types have been performed [173337]. For example, one study examined the diffusion-weighted MRI scan images and performed pathological correlations, but found no significant correlations [33]. While meningothelial meningioma was found to be the most frequent pathological type in our current study, the correlation between cyst type and histopathological type by Nauta et al. [2] showed no statistical significance (p=0.546, Spearman's rho). However, the ratio of meningioma with cyst was conspicuously higher in WHO grade II than in grade I. In the case of grade III meningioma, only one case was histopathologically confirmed, which provided no statistical significance for correlations between grade and histopathological subtype.
In several previous documents regarding meningiomas with cystic lesions, it was recommended for all lesions including cysts to be grossly and totally removed. This is because it is possible that neoplastic cells are present at infiltrations inside the cyst wall, which increases the risk of recurrent cystic meningioma or potential for spreading through CSF [22438]. The study of Liu et al. [21] demonstrated that, it was possible to remove accompanying solid components through cyst decompression in Nauta type III cysts, albeit the gross total removal of the cyst wall was impossible in the case of type II cystic meningioma. There were three cases of recurrence in our study, all of which displayed histopathological progression and were Nauta type IV cystic meningiomas. Two cases started from grade I and II, respectively, and ultimately progressed to WHO grade III. The other case developed from WHO grade I to grade II and was classified as a Nauta type I meningioma with the cyst.
Whether total cystic wall excision is necessary remains unclear. Some authors recommend that excision of the cyst wall is necessary [321], while other authors do not [1339]. In our series, there were three cases of recurrent cystic meningiomas, one of which was intratumoral type, and the others that were removed (Simpson grade III and IV). The degree of resection is a powerful predictor of meningioma recurrence; hence we cannot clearly conclude that cystic wall remnants may cause meningioma recurrence. However, Fortuna et al. [1] insisted that in the case of type II cystic meningiomas, every effort should be made to remove not only the mural nodule but also the cystic wall. Other authors have reported a case of tumor recurrence in type II cystic meningioma in which the cystic wall was not completely removed [9404142], and Boukobza et al. [35] reported that cyst wall components contain cells and therefore, complete removal of cystic components is essential. More prospective studies should be performed to clarify the correlation between complete resection of the cystic component and meningioma recurrence, but if surgical access is feasible, complete removal of the cyst wall may be helpful for preventing recurrence.
Cystic meningioma are rare tumors. Aaccurate diagnosis is made through MRI, and CT is an exclusive diagnosing modality for confirming cystic meningioma. The most common location of cyst-associated meningiomas is the cerebral convexity, and in this case intratumoral type is a relatively common subtype. Although radiologic findings of cyst-type and tumor location had no significant relation to pathological classification, the ratio of cystic meningioma was substantially higher than that of WHO classification grade II; however, this provides no solid ground for concluding a correlation between grade and histopathologic subtype. When surgically feasible, complete removal of the cyst wall may help prevent recurrence.
Figures and Tables
Fig. 1
Nauta type I cyst. Cystic lesion is located at center of tumor. A: MRI with contrast enhancement T1-weighted image of cysts with low signal intensity are observed in the center of the tumor with contrast enhancement. B: T2-weighted image shows high signal intensity, indicating a cystic lesion.
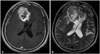
Fig. 2
Nauta type II cyst. The cyst is located within the tumor but it is located at the periphery and the rim of the cystic lesion shows contrast enhancement (A and C). In T2-weighted image, the cystic portion appears as high signal intensity (B).
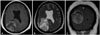
Fig. 3
Nauta type III cyst which does not show any enhancement on the contrast medium (A), is adjacent to the surrounding brain. The mass is part of the cyst wall. The cystic lesion has the isosignal intensity as the cerebrospinal fluid in the ventricle (B).
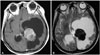
Fig. 4
Nauta type IV cyst which is peritumoral type. A: Tumor located in temporal convexity show enhancement on T1-weighted image with contrast and adjacent cysts are observed. B: The cystic portion shows high signal intensity at T2-weighted image.
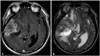
Table 1
Cystic meningioma classification suggested by Nauta et al. [2]

Table 2
Characteristics of the meningiomas

Table 3
Previous cystic meningioma cohorts that is available for Nauta subtype

| Authors | Year | Nauta type | Total | % in intracranial meningioma | Remark | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | Mixed | Undetermined | |||||
| Kolluri et al. [43] | 1987 | 3 | 2 | 1 | 0 | 6 | 6.3 | ||||
| Fortuna et al. [1] | 1988 | 4 | 6 | 8 | 4 | 22 | 1.7 | ||||
| Wasenko et al. [44] | 1994 | 2 | 3 | 1 | 0 | 2 | 8 | 7 | |||
| Sridhar et al. [20] | 1995 | 5 | 4 | 0 | 6 | 2 | 17 | 7.3 | I+IV, I+II | ||
| Zee et al. [7] | 1995 | 6 | 5 | 4 | 0 | 15 | 11.7 | ||||
| Ferrante et al. [45] | 1997 | 2 | 4 | 3 | 0 | 9 | - | ||||
| Chen et al. [33] | 2004 | 3 | 3 | 3 | 1 | 5 | 15 | - | Type V: Worthington's classification | ||
| Jung et al. [36] | 2005 | 8 | 5 | 4 | 1 | 3 (I+III) | 21 | 5.5 | |||
| Souei Mhiri et al. [46] | 2005 | 1 | 1 | 1 | 0 | 1 (II+III) | 4 | - | |||
| Liu et al. [21] | 2007 | 6 | 8 | 2 | 5 | 21 | 3.8 | ||||
| Boukobza et al. [35] | 2016 | 23 | 2 | 3 | 5 | 9 | 1 | 43 | 3.5 | ||
| Current study | 2017 | 15 | 13 | 3 | 7 | 38 | 9.6 | ||||
| Total (%) | 78 (36) | 56 (26) | 33 (15) | 29 (13) | 5 (2) | 15 (7) | 3 (1) | 219 (100) | |||
| Intratumoral | Peritumoral | ||||||||||
| Pinna et al. [26] | 1986 | 8 | 6 | 4 | 18 | 3 cases: no preoperative CT scan | |||||
| Parisi et al. [4] | 1986 | 4 | 3 | 7 | |||||||
| Borovich et al. [47] | 1988 | 0 | 4 | 4 | |||||||
| Kwan et al. [27] | 1992 | 3 | 6 | 9 | |||||||
| Carvalho et al. [48] | 1997 | 3 | 0 | 3 | |||||||
| Weber et al. [49] | 2003 | 1 | 6 | 7 | |||||||
| Zhang et al. [8] | 2009 | 14 | 18 | 32 | |||||||
| Ghani et al. [50] | 2015 | 5 | 8 | 13 | |||||||
| 30 | 45 | 93 | |||||||||
| Cushing et al. [29] | 1938 | N/A | 13 | ||||||||
| Henry et al. [51] | 1974 | N/A | 3 | ||||||||
| Dell et al. [13] | 1982 | N/A | 8 | ||||||||
| el-Fiki et al. [52] | 1996 | N/A | 9 | ||||||||
| el A. Skali et al. [53] | 1998 | N/A | 6 | ||||||||
| Mena et al. [54] | 1998 | N/A | 8 | ||||||||
| Demir et al. [55] | 2007 | N/A | 5 | ||||||||
| Wan et al. [56] | 2010 | N/A | 8 | ||||||||
| 60 | |||||||||||
| Total | 372 | ||||||||||
Table 4
The summary of three cases of recurrent cystic meningiomas

References
1. Fortuna A, Ferrante L, Acqui M, Guglielmi G, Mastronardi L. Cystic meningiomas. Acta Neurochir (Wien). 1988; 90:23–30.

2. Nauta HJ, Tucker WS, Horsey WJ, Bilbao JM, Gonsalves C. Xanthochromic cysts associated with meningioma. J Neurol Neurosurg Psychiatry. 1979; 42:529–535.

3. Odake G. Cystic meningioma: report of three patients. Neurosurgery. 1992; 30:935–940.
4. Parisi G, Tropea R, Giuffrida S, Lombardo M, Giuffrè F. Cystic meningiomas. Report of seven cases. J Neurosurg. 1986; 64:35–38.
5. Rengachary S, Batnitzky S, Kepes JJ, Morantz RA, O'Boynick P, Watanabe I. Cystic lesions associated with intracranial meningiomas. Neurosurgery. 1979; 4:107–114.

7. Zee CS, Chen T, Hinton DR, Tan M, Segall HD, Apuzzo ML. Magnetic resonance imaging of cystic meningiomas and its surgical implications. Neurosurgery. 1995; 36:482–488.

8. Zhang D, Hu LB, Zhen JW, et al. MRI findings of intracranial cystic meningiomas. Clin Radiol. 2009; 64:792–800.

9. Bowen JH, Burger PC, Odom GL, Dubois PJ, Blue JM. Meningiomas associated with large cysts with neoplastic cells in the cysts walls. Report of two cases. J Neurosurg. 1981; 55:473–478.

10. Moraci A, Cioffi F. [Cystic meningioma. An aspect of “forme humide” of Masson]. Neurochirurgie. 1976; 22:701–710.
11. Russell EJ, George AE, Kricheff II, Budzilovich G. Atypical computed tomography features of intracranial meningioma: radiological-pathological correlation in a series of 131 consecutive cases. Radiology. 1980; 135:673–682.

12. Amano K, Miura N, Tajika Y, et al. Cystic meningioma in a 10-monthold infant: case report. J Neurosurg. 1980; 52:829–833.
13. Dell S, Ganti SR, Steinberger A, McMurtry J 3rd. Cystic meningiomas: a clinicoradiological study. J Neurosurg. 1982; 57:8–13.

15. Tapas JN. Intracranial meningioma in a four-month-old infant simulating subdural hematoma. J Neurosurg. 1961; 18:120–121.

16. Lapresle J, Netsky MG, Zimmerman HM. [The pathology of meningiomas; a study of 121 cases]. Am J Pathol. 1952; 28:757–791.
17. Becker D, Norman D, Wilson CB. Computerized tomography and pathological correlation in cystic meningiomas. Report of two cases. J Neurosurg. 1979; 50:103–105.

18. Sigel RM, Messina AV. Computed tomography; the anatomic basis of the zone of diminished density surrounding meningiomas. AJR Am J Roentgenol. 1976; 127:139–141.

19. Paxton R, Ambrose J. The EMI scanner. A brief review of the first 650 patients. Br J Radiol. 1974; 47:530–565.

20. Sridhar K, Ravi R, Ramamurthi B, Vasudevan MC. Cystic meningiomas. Surg Neurol. 1995; 43:235–239.

22. Ambrose J, Gooding MR, Richardson AE. An assessment of the accuracy of computerized transverse axial scanning (EMI scanner) in the diagnosis of intracranial tumour. A review of 366 patients. Brain. 1975; 98:569–582.

23. Claveria LE, Sutton D, Tress BM. The radiological diagnosis of meningiomas, the impact of EMI scanning. Br J Radiol. 1977; 50:15–22.

24. Handa J, Nakano Y, Handa H. Computed tomography in the differential diagnosis of low-density intracranial lesions. Surg Neurol. 1978; 10:179–185.

25. Bonneville F, Sarrazin JL, Marsot-Dupuch K, et al. Unusual lesions of the cerebellopontine angle: a segmental approach. RadioGraphics. 2001; 21:419–438.

26. Pinna G, Beltramello A, Buffatti P, et al. Cystic meningiomas—an update. Surg Neurol. 1986; 26:441–452.

27. Kwan AL, Howng SL, Hwang SL. Cystic intracranial meningioma. Gaoxiong Yi Xue Ke Xue Za Zhi. 1992; 8:591–596.
29. Cushing H, Eisenhardt L. Meningiomas. Their classification, regional behaviour, life history, and surgical end results. Springfield: Charles C Thomas;1938.
30. Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001; 22:1081–1088.
31. Filippi CG, Edgar MA, Uluğ AM, Prowda JC, Heier LA, Zimmerman RD. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001; 22:65–72.
32. Sanverdi SE, Ozgen B, Oguz KK, et al. Is diffusion-weighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012; 81:2389–2395.

33. Chen TY, Lai PH, Ho JT, et al. Magnetic resonance imaging and diffusion-weighted images of cystic meningioma: correlating with histopathology. Clin Imaging. 2004; 28:10–19.

34. Surov A, Ginat DT, Sanverdi E, et al. Use of diffusion weighted imaging in differentiating between maligant and benign meningiomas. A multicenter analysis. World Neurosurg. 2016; 88:598–602.

35. Boukobza M, Cebula H, Pop R, et al. Cystic meningioma: radiological, histological, and surgical particularities in 43 patients. Acta Neurochir (Wien). 2016; 158:1955–1964.

36. Jung TY, Jung S, Shin SR, et al. Clinical and histopathological analysis of cystic meningiomas. J Clin Neurosci. 2005; 12:651–655.

37. Batra A, Tripathi RP. Diffusion-weighted magnetic resonance imaging and magnetic resonance spectroscopy in the evaluation of focal cerebral tubercular lesions. Acta Radiol. 2004; 45:679–688.

39. Maiuri F, Benvenuti D, De Simone MR, Cirillo S, Corriero G, Giamundo A. Cystic lesions associated with meningiomas. Surg Neurol. 1986; 26:591–597.

40. Imagawa K, Nomura T, Asai A, et al. [2 Cases of cystic meningioma]. No Shinkei Geka. 1983; 11:513–518.
41. Lake P, Heiden JS, Minckler J. Cystic meningioma. Case report. J Neurosurg. 1973; 38:638–641.
42. Matsushima T, Kinoshita K, Numaguchi Y, Oda K. [Cystic meningioma--a case report (author's transl)]. No Shinkei Geka. 1978; 6:167–171.
43. Kolluri VR, Reddy DR, Reddy PK, Naidu MR, Devi S. CT-findings in cystic meningiomas. Acta Neurochir (Wien). 1987; 87:31–33.

44. Wasenko JJ, Hochhauser L, Stopa EG, Winfield JA. Cystic meningiomas: MR characteristics and surgical correlations. AJNR Am J Neuroradiol. 1994; 15:1959–1965.
45. Ferrante L, Acqui M, Lunardi P, Qasho R, Fortuna A. MRI in the diagnosis of cystic meningiomas: surgical implications. Acta Neurochir (Wien). 1997; 139:8–11.

46. Souei Mhiri M, Ben Rhouma K, Tlili-Graiess K, et al. [Magnetic resonance imaging features of cystic meningiomas. Report of four cases]. J Neuroradiol. 2005; 32:54–58.
47. Borovich B, Guilburd JN, Doron Y, et al. Cystic meningiomas. Acta Neurochir Suppl (Wien). 1988; 42:147–151.

48. Carvalho GA, Vorkapic P, Biewener G, Samii M. Cystic meningiomas resembling glial tumors. Surg Neurol. 1997; 47:284–289. discussion 289-90.

49. Weber J, Gassel AM, Hoch A, Kilisek L, Spring A. Intraoperative management of cystic meningiomas. Neurosurg Rev. 2003; 26:62–66.

50. Ghani E, Al-Yamany M. Intracranial cystic meningiomas: a rare type of tumours. Br J Neurosurg. 2015; 29:396–400.

51. Henry JM, Schwartz FT, Sartawi MA, Fox JL. Cystic meningiomas simulating astrocytomas. Report of three cases. J Neurosurg. 1974; 40:647–650.
52. el-Fiki M, el-Henawy Y, Abdel-Rahman N. Cystic meningioma. Acta Neurochir (Wien). 1996; 138:811–817.

53. el Abbassi Skalli A, Chikhaoui N, el Hajjam M, Kadiri R. [Cystic meningioma. Apropos of 6 cases]. J Neuroradiol. 1998; 25:275–280.
54. Mena IX, Noboa CA, Leone-Stay G, Vásconez JV, Cárdenas-Mera B. [Cystic meningiomas: unusual forms of intracranial neoplasms]. Rev Neurol. 1998; 27:50–55.
55. Demir MK, Müslüman M, Kilicoglu G, Hakan T, Aker FV. Imaging features of unusual intracranial cystic meningiomas. Can Assoc Radiol J. 2007; 58:109–115.
56. Wan X, Jiang B, Ma Z, Wang J, Hou Y, Liu Y. [Diagnosis and treatment of cystic meningioma]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010; 35:1009–1012.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download