Abstract
Several anatomical variables critically influence therapeutic strategizing for anterior choroidal artery (AChA) aneurysms, and specifically, the safety of flow diversion for these lesions. We review the microsurgical anatomy of the AChA, discussing and detailing these considerations in the treatment of AChA aneurysms, theoretically and in the light of our recent findings.
Consideration of several anatomical features is necessary when treating anterior choroidal artery (AChA) aneurysms and helps to predict the safety of endovascular treatment.22) These variables include length of the communicating internal carotid artery (ICA), choroidal ICA, and pre-bifurcation M1; origin of the AChA in relation to adjacent ICA branches; sidedness of A1 dominance, robustness of flow across the contralateral A1 and anterior communicating artery (ACoA); degree to which AChA-irrigated territory is collaterally-irrigated; and anastomoses of the AChA with nearby vessels.
The AChA was initially described in the 18th century by Vicq D'Azyr. It most commonly originates from the inferolateral posterior ICA wall approximately 2–4 mm distal to the takeoff of the posterior communicating artery (PCoA). A variable number of perforators (none or up to 4) arise between the AChA and PCoA origins, predominantly supplying the laterally-related medial temporal structures, the medially-related optic tract, and the superiorly-related posterior perforated substance. AChA is mono-original in most hemispheres.
The AChA is divided into cisternal and plexal segments. The cisternal AChA averages ~25 mm in length (range, ~18 to 36 mm) and is subdivided by different investigators into two parts.18)23) Rhoton and colleagues18) originally subdivided the cisternal AChA into proximal and distal segments, defined by the lateral geniculate nucleus. A more recent anatomical study uses the natural genu of the AChA to divide the cisternal segment into pre- and post-optic parts, with an average number of 3.4 (range, 1 to 5) and 4.6 (range, 3 to 6) perforators, respectively. The pre- and post-optic cisternal AChA are naturally divided by the genu and most importantly, exhibit a different pattern of perforator course (critical microsurgically) and collateralization of perforator-supplied parenchyma (critical microsurgically and endovascularly).
The initial course of AChA is directed posteromedially following takeoff from the ICA, gently following the semiannular sulcus on the anteromedial surface of the uncus which it faces. After ~8 mm, it then comes to an apex (genu of AChA), diving below and medial to the anterior portion of the optic tract, making a sharp posterolateral turn into the crural cistern, following the posteromedial surface of the uncus, between it laterally, the cerebral peduncle medially, and the continuation of the optic tract superomedially. From the crural cistern, the anterior choroidal artery enters the temporal horn at what is termed the inferior choroidal point, located on average 17.1 mm posterior to temporal limen point (i.e., limen insulae), coursing on the medial aspect of the choroid plexus.19) Interruption of AChA at or distal to this point typically causes no deficits, as all critical AChA-irrigated regions have already been supplied by the cisternal segment of the vessel.
The cisternal AChA supplies a myriad of critical structures, through an average of ~9 perforators (range, 2 to 18 perforators)7)10)12)18) and compromise of this vessel may result in catastrophic stroke. One schema classifies AChA perforators into mesial temporal and deep groups.1)5)7)12)14)23) These small vessels penetrate the visual apparatus, cerebral peduncle, and anterior perforated substance. AChA perforators most commonly supply (in ~50 to 70% of hemispheres) the visual apparatus (optic tract, lateral geniculate nucleus, and optic radiations); pyramidal tract (posterior aspect of posterior aspect of posterior limb of internal capsule (PLIC) and middle third of the cerebral peduncle); and globus pallidus and variably supply (in ~30 to 50% of hemispheres) other basal nuclei (caudate, substantia nigra, and subthalamic nucleus); mesial temporal structures (uncus and amygdala); diencephalon (hypothalamus; superficial part of the ventrolateral thalamic nucleus); and red nucleus.18)
The cisternal perforators of the preoptic part proximal to the genu are directed superolaterally and those distal to the genu (postoptic part) are directed inferomedially.23) Preoptic cisternal perforators supply the optic tract and uncus in 80–90% of hemispheres, and variably supply the anterior perforated substance. Postoptic cisternal perforators supply the optic tract and cerebral peduncle in all hemispheres and variably supply the uncus and lateral geniculate nucleus. Plexal perforators supply the choroid plexus principally.23) The distal-most branch of the cisternal AChA is the capsulothalamic artery, supplying parenchyma mediating vision (lateral geniculate nucleus, optic radiations, and retrolenticular internal capsule) and motor control (PLIC).12)
Collateralization of AChA-irrigated parenchyma is extant and interruption of blood supply is effectively compensated for in many cases of acute occlusion. Thus, with flow diverter (FD) treatment, in cases in which occlusion should occur, this would be gradual and effectively compensated by recruitment of pre-existent, and de novo development of, collateral supply and the formation of anastomoses. Thus, the state of vascular anatomy in this region renders FD a safe approach for the treatment of AChA aneurysms, leaving the major anatomic determinants of safety and efficacy for the same 1) distance of AChA from PCoA and ICA bifurcation; 2) PCoA size/dominance; 3) M1 length and lenticulostriate location/density; and 4) ipsilateral and contralateral A1 robustness/dominance and cross-flow.
The A1 segments of the anterior cerebral artery (ACA) communicate across the midline via the ACoA and provide a variable number of perforators (1–12) to the optic nerve and chiasm, anterior hypothalamus, striatum, anterior commissure, and forniceal pillars.15) A1 may, on occasion, give rise to the recurrent artery of Heubner. It continues as the A2 segment and supplies the medial cerebral structures anterior to PCA-irrigated territory.
In order to treat an AChA aneurysm with flow diversion, the stent is placed across the AChA and A1 origins, passing from choroidal ICA into proximal M1. Crossing of the A1 origin by a FD stent carries the theoretical risk of ischemic infract. If the crossed A1 is dominant, there is a higher risk of neurologic deficit with occlusion, but also a higher likelihood of maintained patency given higher flow demand. Conversely, occlusion of a non-dominant A1 is less likely to result in neurologic deficit, but the lower flow deems maintained patency less likely. The preferability of the former versus the latter case awaits empirical evaluation of the stated theoretical arguments on a large scale.
FD treatment of AChA aneurysms would be most strongly contraindicated in the case of a dominant ipsilateral A1 with minimal cross-flow across ACoA from the contralateral A1; however, an especially diminutive or dominant A1 is associated with larger ACoA, since it is well-established that the size of the ACoA increases in direct proportion to left-right A1 caliber difference,15) rendering this situation unlikely.
The AChA is typically a mono-original mono-luminal vessel arising off the ICA distal to the PCoA (Figs. 1 & 2). Variant origins of the AChA include a mono-original vessel with early bifurcation into two separate vessels, a double-origin vessel arising off the ICA, or an origin from ICA bifurcation, PCoA, or M1.2)6)13)18)23) Importantly, a true duplicate AChA should not be confused with a C4-origin vessel terminating in the uncus, which is an aberrant uncal artery, and is unlikely to result in deficit with gradual vessel occlusion consequent to jailing by FD. M1 and PCoA-origin AChA aneurysms are less safely treated via FD.
Distance between AChA and PCoA and ICA bifurcation averages 4 mm (range, 1.5 to 9 mm) and 5.6 mm (range, 2.5 to 10 mm), respectively.9) Greater distance between AChA and PCoA origins in theory permits safer placement of FD across an AChA aneurysm without occluding PCoA. However, in practice, the PCoA is almost always crossed when treating AChA aneurysms with FDs.
The greater the length from PCoA to ICA bifurcation, the less of FD that must be situated in M1. On average, the summed length of the communicating and choroidal segments averages approximately 9-10 mm, requiring, in most cases, at least 7 mm of the FD device to be placed in M1. Thus, for AChA aneurysms, FDs are placed in the supraclinoid ICA to M1 across the A1 origin. According to Rhoton's classification, the M1 segment of the middle cerebral artery (MCA) contains the first bifurcation/trifurcation of this vessel.8) Thus, pre-bifurcation M1 length serves as a critical consideration in treating AchA aneurysms, so as to avoid intrusion of the FD into the MCA bifurcation. M1 gives off ~10 (range, 1 to 21) superoposteriorly-directed lenticulostriates, most of which arise from its proximal aspect, penetrating the anterior perforated substance to supply anterior basal ganglia, internal capsule, and anterior commissure20) and there remains a theoretical risk of stroke with FD-related jailing.
In a series of 50 patients with Parkinson's disease,3)4) double occlusion of AChA at the origin and immediately after the branches to the globus pallidus effectively cured tremor frequently, with only 6% of patients developing hemiplegia. In a much smaller series of 5 patients, only one person developed hemiplegia.16) These studies were the first to provide evidence that AChA-irrigated parenchyma can be salvaged by other vessels and anastomoses. Collateral supply to AChA territory is provided by direct perforators from the C4 segment of the ICA, PCoA, and PCA. C4 perforators may supply the optic tract, cerebral peduncle, anterior perforated substance, uncus, and hypothalamus.
The size relationship of the AChA to PCoA is inverse — the smaller the AChA, the greater the contribution of PCoA supply to PLIC and the larger the AChA, the lesser contribution of PCoA to the genu and anterior limb of the internal capsule. This is analogous to the AChA-PCA size relationship. The more diminutive the AChA, the greater the PCA supply to the mesencephalic structures (cerebral peduncle, substantia nigra, and red nucleus), optic tract, and lateral geniculate nucleus. Anastomoses between AChA and PCA which occurs in ~50% of hemispheres also contribute to salvage of AChA-irrigated parenchyma in the case of occlusion, These occur within the substance of the choroid plexus, via the lateral posterior choroidal artery and on the medial and lateral surfaces of the uncus and lateral geniculate nucleus, respectively.
Preoptic cisternal AChA-irrigated parenchyma is well-collateralized in the anterior aspect of the optic tract by PCoA, medial PChA, and leptomeningeal vessels2)16)24) and in mesial temporal structures by ICA and MCA collaterals.7)25) The fate of postoptic cisternal AChA-irrigated parenchyma (optic radiations and posterior tract, PLIC and cerebral peduncle) is less robustly protected rendering infarct more likely in this region in the case of occlusion, though PCA-AChA anastomoses in choroid plexus via lateral PChA may provide adequate collaterization.
FD of distal AChA aneurysms is unlikely to be effective, given extensive anastomoses distally that would fill the lesion retrogradely. These lesions may be coil-embolized and AChA aneurysms distal to the inferior choroidal point may safely be secured by sacrificing the vessel.11) When endovascular techniques are deemed risky or ineffective, microsurgical access to the pre- and postoptic cisternal AChA can be achieved via transsylvian and trans-temporal (or trans-insular)/transchoroidal approaches, respectively.23) Great care must be taken with exposure of the postoptic cisternal AChA while incising the uncus given the great density of critical vascular elements (PCA, medial PChA, AChA, and basal vein of Rosenthal) in the crural cistern.
FD treatment of AChA origin aneurysms is rendered less safe with close proximity of the PCoA origin to AChA, short length of choroidal and communicating ICA segments and prebifurcation M1, absence of cross-flow across the ACoA (especially if ipsilateral A1 is dominant), a large dominant AChA, a diminutive PCoA; and diminituve AChA-collateralizing/anastomosing vessels. FD treatment is safer when PCoA is diminutive, non-fetal, and far away from ACoA, with greater length of communicating, choroidal, and pre-bifucation M1 segments, and with greater distance between PCoA origin and the intra-M1 segment bifurcation.
Figures and Tables
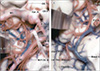 | Fig. 1Anterior choroidal artery course and relations viewed from inferiorly. (A) The right AChA arises from the posterior wall of the ICA above the origin of the PCoA and passes backward below the optic tract and lateral to the PCA. It ascends around the medial surface of the uncus as it travels posteriorly. (B) The posterior uncal segment has been retracted. The AChA passes above the posterior uncal segment and enters the temporal horn by passing through the choroidal fissure located between the thalamus above and fimbria of the fornix below. The lateral geniculate body forms the part of the thalamus above where the artery enters the choroidal fissure. The dentate gyrus is located at the lower edge of the fimbria. Reprinted with permission from reference 17. Olf. Tr. = FULL NAME; M1 = sphenoidal segment of middle cerebral artery; Car. A. = internal carotid artery; CN II = optic nerve; Lent. Str. A. = lenticulostriate arteries; P.Co.A = posterior communicating artery; Optic Tr. = optic tract; CN III = oculomotor nerve; P1 = pre-communicating segment of posterior cerebral artery; A.Ch.A = anterior choroidal artery; P2 = post-communicating segment of posterior cerebral artery; M.P.Ch.A = medial posterior choroidal artery; P.C.A. = posterior cerebral artery; Dent. Gyr. = dentate gyrus; Chor. Fiss. = choroidal fissure; Lat. Gen. Body = lateral geniculate body; Basal V = basal vein of Rosenthal; ICA = internal carotid artery. |
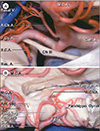 | Fig. 2Anterior choroidal artery course and relations viewed from laterally and medially. (A) Lateral view. The right AChA arises above the origin of the PComA and passes upward and backward around the uncus to reach the temporal horn. (B) Medial view. The AChA pursues an angulated course, descending along the anterior segment of the uncus, but at the uncal apex, it turns sharply upward, reaching the upper part of the posterior uncal segment before entering the temporal horn. Reprinted with permission from reference 17. Basal V. = basal vein of Rosenthal; M.C.A. = middle cerebral artery; A.Ch.A. = anterior choroidal artery; P.Co.A = posterior communicating artery; Car. A. = internal carotid artery; S.C.A. = FULL NAME; CN III = oculomotor nerve; cating segment of anterior cerebral artery; Bas. A. = basilar artery; Temp. Horn = temporal horn of lateral ventricle; Ant. Seg. = anterior segment of uncus; A1 = pre-communicating segment of anterior cerebral artery; Parahippo. Gyrus = parahippocampal gyrus; Post. Seg. = posterior segment of uncus; P2 = second segment of posterior cerebral artery; PComA = FULL NAME. |
References
1. Abbie AA. The clinical significance of the anterior choroidal artery. Brain. 1933; 09. 56:233–246.

2. Carpenter MB, Noback CR, Moss ML. The anterior choroidal artery; its origins course, distribution, and variations. AMA Arch Neurol Psychiatry. 1954; 06. 71(6):714–722.
3. Cooper IS. Surgical alleviation of Parkinsonism; effects of occlusion of the anterior choroidal artery. J Am Geriatr Soc. 1954; 11. 2(11):691–718.

4. Cooper IS. Surgical occlusion of the anterior choroidal artery in Parkinsonism. Surg Gynecol Obstet. 1954; 08. 99(2):207–219.
5. Decroix JP, Graveleau P, Masson M, Cambier J. Infarction in the territory of the anterior choroidal artery. A clinical and computerized tomographic study of 16 cases. Brain. 1986; 12. 109(Pt 6):1071–1085.
6. Erdem A, Yaşargil G, Roth P. Microsurgical anatomy of the hippocampal arteries. J Neurosurg. 1993; 08. 79(2):256–265.

7. Fernández-Miranda JC, de Oliveira E, Rubino PA, Wen HT, Rhoton AL Jr. Microvascular anatomy of the medial temporal region: part 1: its application to arteriovenous malformation surgery. Neurosurgery. 2010; 09. 67:3 Suppl Operative. ons237–ons276. discussion ons276.

8. Gibo H, Carver CC, Rhoton AL Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981; 02. 54(2):151–169.

9. Gibo H, Lenkey C, Rhoton AL Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981; 10. 55(4):560–574.

10. Huther G, Dörfl J, Van der Loos H, Jeanmonod D. Microanatomic and vascular aspects of the temporomesial region. Neurosurgery. 1998; 11. 43(5):1118–1136.

11. Inci S, Arat A, Ozgen T. Distal anterior choroidal artery aneurysms. Surg Neurol. 2007; 01. 67(1):46–52. discussion 52.

12. Marinković S, Gibo H, Brigante L, Nikodijević I, Petrović P. The surgical anatomy of the perforating branches of the anterior choroidal artery. Surg Neurol. 1999; 07. 52(1):30–36.

13. Morandi X, Brassier G, Darnault P, Mercier P, Scarabin JM, Duval JM. Microsurgical anatomy of the anterior choroidal artery. Surg Radiol Anat. 1996; 18(4):275–280.

14. Nelles M, Gieseke J, Flacke S, Lachenmayer L, Schild HH, Urbach H. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol. 2008; 03. 29(3):488–493.

15. Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976; 09. 45(3):259–272.

16. Rand RW, Brown WJ, Stern WE. Surgical occlusion of the anterior choroidal arteries in Parkinsonism; clinical and neuropathologic findings. Neurology. 1956; 06. 6(6):390–401.
18. Rhoton AL Jr, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979; 08. 12(2):171–187.
19. Ribas EC, Yagmurlu K, Wen HT, Rhoton AL Jr. Microsurgical anatomy of the inferior limiting insular sulcus and the temporal stem. J Neurosurg. 2015; 06. 122(6):1263–1273.

20. Rosner SS, Rhoton AL Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg. 1984; 09. 61(3):468–485.

21. Saeki N, Rhoton AL Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. 1977; 05. 46(5):563–578.

22. Srinivasan VM, Ghali MGZ, Cherian J, Mokin M, Puri AS, Grandhi R, et al. Flow diversion for anterior choroidal artery (AChA) aneurysms: a multi-institutional experience. J Neurointerv Surg. 2017; 10. 31. [Epub ahead of print].

23. Tanriover N, Kucukyuruk B, Ulu MO, Isler C, Sam B, Abuzayed B, et al. Microsurgical anatomy of the cisternal anterior choroidal artery with special emphasis on the preoptic and postoptic subdivisions. J Neurosurg. 2014; 05. 120(5):1217–1228.

24. van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg. 1992; 12. 77(6):927–940.

25. Yaşargil MG. Anterior choroidal artery. In : Yaşargil MG, editor. Microsurgical Anatomy of the Basal Cisterns and Vessels of the Brain, Diagnostic Studies, General Operative Techniques and Pathological Considerations of the Intracranial Aneurysms. Stuttgart: Georg Thieme Verlag;1984. p. 66–70.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download