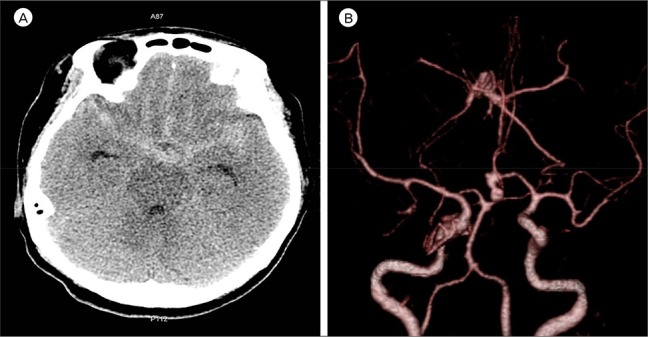
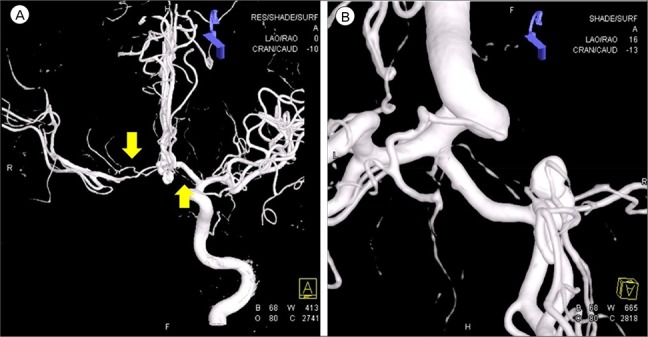
The RAH exhibited a mean outer diameter of 0.8 ± 0.04 mm (range, 0.2–1.5) and a mean length of 23.4 ± 1.1 mm (range, 12–38). The origin of the RAH occurs most commonly at the junction of the ACA and ACoA, then from the proximal A2 segment, and finally from the distal A1 segment.
4)7)9) The RAH supplies parts of the caudate nucleus, basal ganglia, and anterior limb of the internal capsule.
9)
There are only a few papers in the literature that specifically describe the territory affected by RAH infarction.
1)3) RAH occlusion can manifest as various symptoms, including weakness of the contralateral face and arm without sensory loss, transient aphasia, dysarthria, cognitive and behavioral abnormalities, abulia, agitation, hyperactivity, contralateral neglect, obsessive-compulsive disorder, and memory dysfunction (i.e., poor ability to perform declarative and procedural memory tasks). However, most RAH infarctions are accompanied by only transient neurological deficits, if any.
1)2)10)11)12) The possible causes of cerebral infarction following SAH are cerebral vasospasm, microthrombo-embolism, cortical spreading ischemia, spasm in the microcirculation and impaired cerebral autoregulation. Operative maneuvers that can lead to blood flow disturbance and consequent infarction include inappropriate aneurysmal neck clipping, temporary occlusion of the parent artery, direct injury, retraction with a spatula, or trapping of the parent artery.
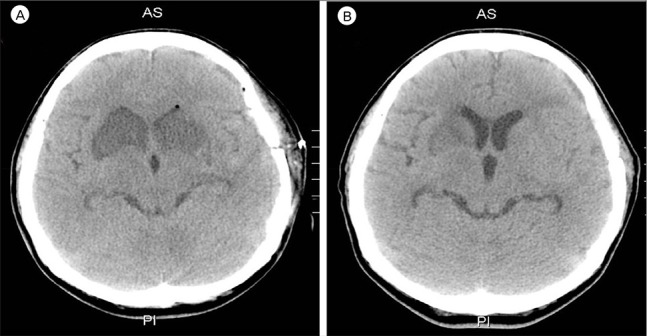
12) In this case, multiple factors are considered to have caused infarction of the bilateral RAH territory, including the SAH corresponding to a Fisher Grade III, placement five times of temporary clips on the A1 segment, hypotension during surgery (systolic blood pressure less than 100 mmHg), and a mean arterial pressure of 65 mmHg. Cerebral vasospasm was also a significant causative factor in this case. The mechanism of vasospasm remains poorly understood. However, in the last 3 decades, certain mechanisms have been identified that might play a role in its development. Factors such as calcium-dependent and -independent vasoconstriction, breakdown of products in the blood of subarachnoid space, and imbalance between vasoconstrictor and vasodilator substances derived from the endothelium may be major causes of vasospasm.
8) Cerebral vasospasm post-SAH may involve the complete arterial system from the circle of Willis up to the distal vessel segments.
15) A high volume of cisternal hemorrhage on an admission CT scan in a patient with Fisher Grade III aneurysmal SAH was found to be highly associated with delayed ischemic neurological deficit (DIND) due to cerebral vasospasm.
6) When the subarachnoid blood went undetected or was distributed diffusely, severe vasospasm was almost never encountered; however, in the presence of a large amount of SAH, severe vasospasm almost always occurred.
5) Approximately 11–20% of episodes of DIND are characterized by cerebral infarction.
13)14) Cerebral infarction mostly occurs in patients who develop angiographic vasospasm.
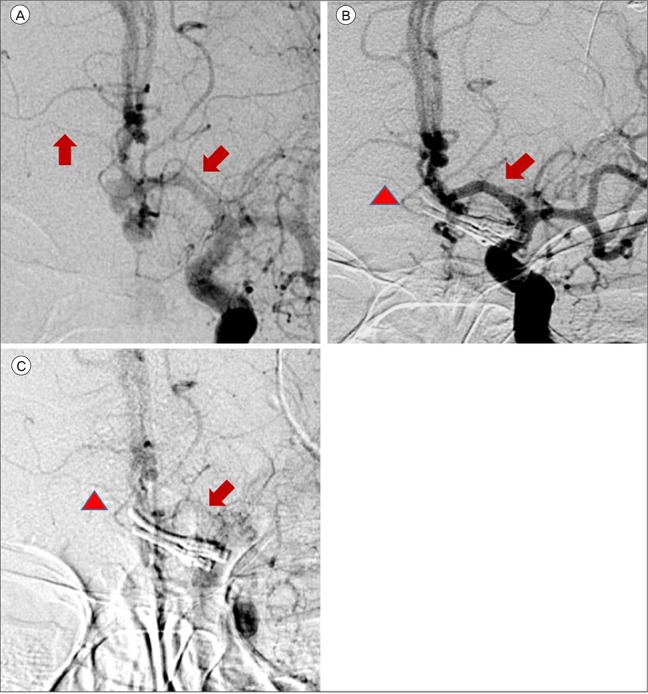
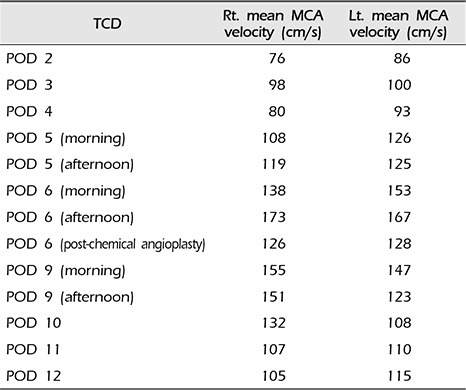
14) Our patient was alert during her stay in the neuro-intensive care unit. As the mean MCA velocity increased, the patient became lethargic; this was attributed to DIND. Angiographic vasospasm was found in the preoperative and postoperative TFCA. Thus it was deduced that the decreased perfusion of both RAH due to cerebral vasospasm was a major cause of the bilateral RAH territorial infarction. In addition to cerebral vasospasm, the other causes of bilateral RAH infarction were assumed to be the repeated temporary clipping on the Lt. A1 segment and the presence of hypotension during surgery. The prolonged temporary clipping time of 26 minutes, 45 seconds and hypotension (systolic blood pressure less than 100 mmHg for 115 minutes and mean arterial pressure of 65 mmHg for 70 minutes) during surgery likely significantly lowered cerebral blood perfusion and cerebral perfusion pressure. These conditions would have been sufficient to cause bilateral RAH infarction. During the temporary clipping, the systolic blood pressure was always below 100 mmHg; the mean arterial blood pressure was below 65 mmHg for half of the clipping time. Temporary clipping of the main cerebral artery can lead to infarcts of the perforating artery, and temporary clipping of the A1 segment can lead to infarction of the RAH.
12) Sasaki et al.
12) reported nine patients of RAH infarction following aneurysm clipping, all of whom had ACoA aneurysm, in a population of 1,045 patients with treated aneurysms and 238 with ACoA aneurysms. The incidence of the causes of RAH infarctions: A1 temporary occlusion (four), retraction (three) and injury of RAH (two). In this case, vasospasm was considered to be a more likely cause of RAH infarction than temporary occlusion or hypotension. This inference was made based on the fact that immediately following the operation the patient had no neurologic deficit or abnormal mental status. The patient's reduced mental status appeared alongside the increase in the mean MCA velocity. There were no abnormal findings in the post-operative brain CT. An intra-operative Doppler was applied and intact flow of the RAH was observed.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download