Abstract
An intracranial pseudoaneurysm (PA) is a very rare disease and is known to occur in less than 1% of intracranial aneurysms. The pathophysiology and the modality of the proper treatment of PA have not yet been clearly established. We report a case of PA associated with ruptured cerebral aneurysms which was successfully treated by coil embolization, and also discuss the possible hypothesis on the formation of the PA and feasibility of endovascular treatments.
An intracranial pseudoaneurysm (PA) is a very rare disease and is known to occur in less than 1% of intracranial aneurysms.2) However, an intracranial PA associated with rupture of a true aneurysm has rarely been reported, and its incidence rate is unknown. Moreover, the pathophysiology and the modality of the proper treatment have not yet been clearly established. Herein, we report a case of PA associated with ruptured cerebral aneurysm which was successfully treated by coil embolization, and also discuss the possible hypothesis on the formation of the PA and feasibility of endovascular treatments.
A 67-year-old female with history of hypertension and diabetes mellitus presented with sudden deterioration of conscious level.
Non-contrast computed tomography (CT) showed high density within the basal cistern (Fig. 1A). CT angiogram showed a posterior communicating artery (PcomA) aneurysm with an elongated irregular shape (neck/height/width 4.84/7.73/4.59 mm) (Fig. 1B). The clinical and radiographic presentation of the patient was consistent with high-grade aneurysmal subarachnoid hemorrhage (SAH). While waiting for an external ventricular drainage (EVD) to reduce the intracranial pressure, she had a generalized seizure. The follow-up CT scan showed an increased amount of hyperdensity within the whole cistern compared to the previous CT scan due to rebleeding (Fig. 1C).
To control the intracranial pressure, she underwent an urgent EVD on the right side for drainage of the cerebrospinal fluid. The patient's conscious level improved within a few hours after placing the EVD. The patient was transferred to the interventional suit for digital subtracted angiography (DSA) and coil embolization. DSA showed a PA arising from the previously ruptured PcomA aneurysm which did not observed on the initial CTA a few hours earlier. DSA demonstrated an irregular, distorted aneurysm at the end of the PcomA, measuring 4.5 mm (Fig. 1D). This lesion filled with contrast unevenly in a delayed fashion, as shown in the early and late arterial phases. Contrast stasis and delayed contrast washout were also observed in the early and late venous phase (Fig. 1E). These findings suggested the possibility of a PA. Under fluoroscopic supervision, a SL-10 microcatheter (Stryker, Fremont, CA, USA) was advanced over a Synchro II microwire (Stryker) to access the aneurysm. Due to high risk of intraoperative rebleeding, the microcatheter was purposely located near the aneurysmal neck for delivery of coils only to the base of the true aneurysm and not to the PA (Fig. 1F). Coils were intentionally packed in the proximal true aneurysm to prevent any irritation of the PA. Both the aneurysm and the PA were still opacified after the first frame coil, however, the PA was gradually obliterated after additional coil delivery. Only the true aneurysmal sac was filled with multiple coils, then a few loops of coil were located in the PA, but there was no evidence of rebleeding (Fig. 1G). Post procedural angiogram showed no contrast filling in either the aneurysm or the PA.
Occurrence of a PA after intracranial aneurysm rupture is very rare,1)4)5) and it is known to be extremely fragile, rebleeding often occurs before or during the treatment procedure. According to the previous report, it showed poorer clinical outcomes than a typical aneurysm without PA.5) For such a lesion, coil embolization also showed a very high risk of rebleeding. Lempert et al.3) reported that rupture during endovascular treatment occurred in three of four patients with PA associated with aneurysmal SAH. Thus, endovascular treatments for such lesions require other special interventional techniques in addition to conventional coiling techniques. For proper treatment of these lesions, physicians should be aware of the angiographic characteristics of PAs. Fortunately, these lesions often have characteristic angiographic findings, including abnormal contrast medium filling, with an irregular aneurysm wall and delayed opacification compared with a true aneurysmal sac, and delayed washout of contrast medium from the irregularly shaped portion of the aneurysm and retention of contrast medium even during the venous phases were observed on angiography.5) These angiographic findings were often referred to as ‘ghost aneurysm’ or ‘snowman's head’.1)4)
PAs have developed from the gradually reorganizing clot surrounding the site of rupture of the true aneurysm, and during the course, the blood clot covering the rupture site of the true aneurysm may have been dissolved from the inside by fibrinolysis, resulting in gradual enlargement of the PA cavity.1) This process occurs relatively slowly within the pulsatile subarachnoid space. And without rebleeding, PA formation only within the pulsatile hematoma, the patient does not show deterioration. And, if the diagnostic DSA is performed in this state, a typical ‘ghost aneurysm’ or ‘snowman's head’ will be seen.
However, in our case, the patients neurological condition worsened due to rebleeding and the PA was observed, initially it was not seen in CT angiogram. Therefore, based only on the theory of intrinsic clot lysis pathways, it is difficult to explain this urgent rebleeding and PA formation in case. We assume that tearing of the hematoma present in the subarachnoid space at the moment rebleeding occurs could create an empty space (PA), and this empty space would be a PA. This empty space has a hole through the normal cerebral vessel without a true vessel wall. Because it does not have a true vessel wall that supports the hemodynamic stress and it has a hole through the normal cerebral vessel, it can be very easily re-ruptured at any time. In particular, when planning a coil embolization, rebleeding may occur due to advancement of the microcatheter and coils. Therefore, during endovascular treatment, due to the very fragile nature of the PA wall, the physician should be cautious to avoid advancing microinstruments into the area of the PA. In other words, we should do that the coil delivered almost to the proximal true part of the aneurysm.
Figures and Tables
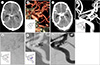 | Fig. 1Image collections of the case. (A) CT shows high density on the basal cistern. (B) CT angiogram shows a PcomA aneurysm with an elongated irregular shape. (C) A CT scan after rebleeding shows massive higher density. (D) 3D DSA shows a PA (arrow) arising from the previously ruptured PcomA aneurysm which did not observed on the initial CT (image B). (E) Delayed washout of contrast medium from the irregularly shaped portion of the aneurysm during the venous phases. (F) Frame coil delivered almost to the proximal true part of the aneurysm. (G) Post procedural angiogram shows no contrast filling within the aneurysm or PA. CT = computed tomography; PcomA = posterior communicating artery; DSA = digital subtraction angiography; PA = pseudoaneurysm. |
References
1. Ide M, Kobayashi T, Tamano Y, Hagiwara S, Tanaka N, Kawamura H. Pseudoaneurysm formation at the rupture site of a middle cerebral artery aneurysm--case report. Neurol Med Chir (Tokyo). 2003; 09. 43(9):443–446.
2. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg. 1990; 07. 73(1):18–36.
3. Lempert TE, Halbach VV, Higashida RT, Dowd CF, Urwin RW, Balousek PA, et al. Endovascular treatment of pseudoaneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol. 1998; 05. 19(5):907–911.
4. Mori K, Kasuga C, Nakao Y, Yamamoto T, Maeda M. Intracranial pseudoaneurysm due to rupture of a saccular aneurysm mimicking a large partially thrombosed aneurysm (“ghost aneurysm”): radiological findings and therapeutic implications in two cases. Neurosurg Rev. 2004; 10. 27(4):289–293.

5. Nomura M, Kida S, Uchiyama N, Yamashima T, Yoshikawa J, Yamashita J, et al. Ruptured irregularly shaped aneurysms: pseudoaneurysm formation in a thrombus located at the rupture site. J Neurosurg. 2000; 12. 93(6):998–1002.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download