Abstract
Purpose
We investigated the extent of adenosine A1 agonist-induced expression and regulation of matrix metalloproteinase 2 (MMP-2) synthesis in human trabecular meshwork cells (HTMC).
Methods
Primary HTMC cultures were exposed to 0.1 or 1.0 µM N6-cyclohexyladenosine (CHA) for 2 h in the presence or absence of an inhibitor thereof, 8-cyclopentyl-1,3-dimethylxanthine (CPT). The expression level of mRNA encoding MMP-2 was assessed via reverse transcription-polymerase chain reaction, and the levels of tissue inhibitor of metalloproteinase 2 (TIMP2) and membrane-type-1 MMP (MT1-MMP) measured by Western blotting. The permeability of the HTMC monolayer was assessed with the aid of carboxyfluorescein.
Results
CHA at 1.0 µM increased the permeability of the HTMC monolayer (p = 0.003) and CHA at both 0.1 and 1.0 µM significantly increased MMP-2 mRNA expression, which was inhibited by co-exposure to CPT (all p < 0.05). CHA increased MMP-2 activity, decreased that of TIMP2, and increased that of MT1-MMP (all p < 0.05).
Figures and Tables
 | Figure 1Exposure to 1.0 µM N6-cyclohexyladenosine (CHA) increased the permeability of carboxyfluorescin significantly compared to control. Exposure to the 10 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) decreased permeability significantly when co-exposed to 0.1 or 1.0 µM CHA (*). When co-exposed to CPT, 0.1 or 1.0 µM CHA decreased permeability significantly compared with exposure to CHA alone (**). Carboxyfluorescin intensity of outer chamber normalized to the mean value obtained using non-exposed control (permeability 100%). *,**p < 0.05. |
 | Figure 2Exposure to 0.1, 1.0 µM N6-cyclohexyladenosine (CHA) increased significantly the expression of matrix metalloproteinase 2 (MMP2) mRNA with or without co-exposure to 10 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) compared to control. Co-exposure to the 10 µM CPT with 0.1 or 1.0 µM CHA decreased the expression of MMP2 mRNA compared to exposure to CHA alone, respectively. β-actin used as internal standard. *p < 0.05. |
 | Figure 3Exposure to 0.1, 1.0 µM N6-cyclohexyladenosine (CHA) increased significantly the activity of matrix metalloproteinase 2 with or without co-exposure to 10 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) compared to control. GAPDH used as internal standard. *p < 0.05. |
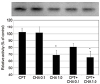 | Figure 4Exposure to 1.0 µM N6-cyclohexyladenosine (CHA) decreased significantly the activity of tissue inhibitor of metalloproteinase 2 (TIMP2) with or without co-exposure to 10 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) compared to control. Exposure to the 10 µM CPT alone did not affect the activity of TIMP2. GAPDH used as internal standard. *p < 0.05. |
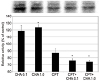 | Figure 5Exposure to 1.0 µM N6-cyclohexyladenosine (CHA) increased significantly the activity of membrane-type-1 matrix metalloproteinase (MT1-MMP). Co-exposure to the 10 µM 8-cyclopentyl-1,3-dimethylxanthine (CPT) with 0.1 or 1.0 µM CHA decreased the activity of MT1-MMP. GAPDH used as internal standard. *p < 0.05. |
Notes
References
1. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984; 91:564–579.

2. Rohen JW, Lütjen-drecoll E, Flügel C, et al. Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp Eye Res. 1993; 56:683–692.
3. Schmidl D, Schmetterer D, Garhöfer G, Popa-Cherecheanu A. Pharmacotherapy of glaucoma. J Ocul Pharmacol Ther. 2015; 31:63–77.

4. Kopczynski CC, Epstein DL. Emerging trabecular outflow drugs. J Ocul Pharmacol Ther. 2014; 30:85–87.

5. Zhong Y, Yang Z, Huang WC, Luo X. Adenosine, adenosine receptors and glaucoma: an updated overview. Biochimica et Biophysica Acta. 2013; 1830:2882–2890.

6. Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48:2105–2114.

7. Shearer TW, Crosson CE. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43:3016–3020.
8. Kim JW. Comparative study of the effects of trabecular meshwork outflow drugs on the permeability and nitric oxide production in trabecular meshwork cells. Korean J Ophthalmol. 2017; 31:452–459.

9. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.

10. Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005; 46:2424–2432.

11. Kameda T, Inoue T, Inatani M, et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm’s canal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53:3092–3103.

12. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–1049.
13. Alvarado JA, Wood I, Polansky JR. Human trabecular cells. II. Growth pattern and ultrastructural characteristics. Invest Ophthalmol Vis Sci. 1982; 23:464–478.
14. Crosson CE. Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther. 1995; 273:320–326.
15. Crosson CE. Intraocular pressure responses to the adenosine agonist cyclohexyladenosine: evidence for a dual mechanism of action. Invest Ophthalmol Vis Sci. 2001; 42:1837–1840.
16. Tian B, Gabelt BT, Crosson CE, Kaufman PL. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp Eye Res. 1997; 64:979–989.

17. Husain S, Shearer TW, Crosson CE. Mechanisms linking adenosine A1 receptors and extracellular signal-regulated kinase 1/2 activation in human trabecular meshwork cells. J Pharmacol Exp Ther. 2007; 320:258–265.

18. Fleischhauer JC, Mitchell CH, Stamer WD, et al. Common actions of adenosine receptor agonists in modulating human trabecular meshwork cell transport. J Membrane Biol. 2003; 193:121–136.

19. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88:676–682.

20. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39:2649–2658.
21. Pang IH, Helberg PE, Fleenor DL, et al. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:3485–3493.

22. Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003; 59:812–823.

23. Löffek S, Schilling O, Franzke CW. Series “matrix metalloproteinases in lung health and disease”: biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011; 38:191–208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download