Abstract
We present image findings, especially rare MRI of a primary breast angiosarcoma with its histopathology, and also analyze the relevant medical literature reports in terms of the MRI findings. As our patient had unique features of a primary breast angiosarcoma, this case could be very helpful for future diagnosis of this rare breast malignancy by MRI.
References
1. Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcomas: imaging findings in 24 patients. Radiology. 2007; 242:725–734.

2. Dashevsky BZ, Charnoff-Katz K, Shin SJ, Babagbemi K, Rosenblatt R. A case of primary breast angiosarcoma. Radiol Case Rep. 2013; 8:741.

3. Lin WM, Juan YH, Lin YC, Ueng SH, Lo YF, Cheung YC. Awareness of primary spontaneous hemorrhagic angiosarcoma of the breast associated with Kasabach-Merritt syndrome in a pregnant woman by enhanced magnetic resonance imaging: a CARE-compliant case report. Medicine (Baltimore). 2016; 95:e5276.
4. Wang L, Lao IW, Yu L, Yang W, Wang J. Primary breast angiosarcoma: a retrospective study of 36 cases from a single Chinese medical institute with clinicopathologic and radiologic correlations. Breast J. 2017; 23:282–291.

6. Georgiannos SN, Sheaff M. Angiosarcoma of the breast: a 30 year perspective with an optimistic outlook. Br J Plast Surg. 2003; 56:129–134.

7. Bennani A, Chbani L, Lamchahab M, et al. Primary angiosarcoma of the breast: a case report. Diagn Pathol. 2013; 8:66.

8. Bhosale SJ, Kshirsagar AY, Patil MV, Wader JV, Nangare N, Patil PP. Primary angiosarcoma of breast: a case report. Int J Surg Case Rep. 2013; 4:362–364.

9. Liberman L, Dershaw DD, Kaufman RJ, Rosen PP. Angiosarcoma of the breast. Radiology. 1992; 183:649–654.

10. Marchant LK, Orel SG, Perez-Jaffe LA, Reynolds C, Schnall MD. Bilateral angiosarcoma of the breast on MR imaging. AJR Am J Roentgenol. 1997; 169:1009–1010.

11. Kim YS, Kim YJ, Yim KI, Park WC. A case report of primary breast angiosarcoma with fatal pulmonary hemorrhage due to thrombocytopenia. J Korean Surg Soc. 2012; 82:251–255.

12. Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999; 211:101–110.

13. Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol. 2009; 13:147–150.

14. Glazebrook KN, Morton MJ, Reynolds C. Vascular tumors of the breast: mammographic, sonographic, and MRI appearances. AJR Am J Roentgenol. 2005; 184:331–338.

15. O'Neill AC, D'Arcy C, McDermott E, O'Doherty A, Quinn C, McNally S. Magnetic resonance imaging appearances in primary and secondary angiosarcoma of the breast. J Med Imaging Radiat Oncol. 2014; 58:208–212.
Fig. 1.
Mammography (a) shows extremely dense breast. Sonography (b) shows irregular indistinct heterogeneous echoic mass of the upper inner quadrant of the left breast. The increased vascularity in the hypoechoic portion of the scanned area is demonstrated by color Doppler scanning (c).
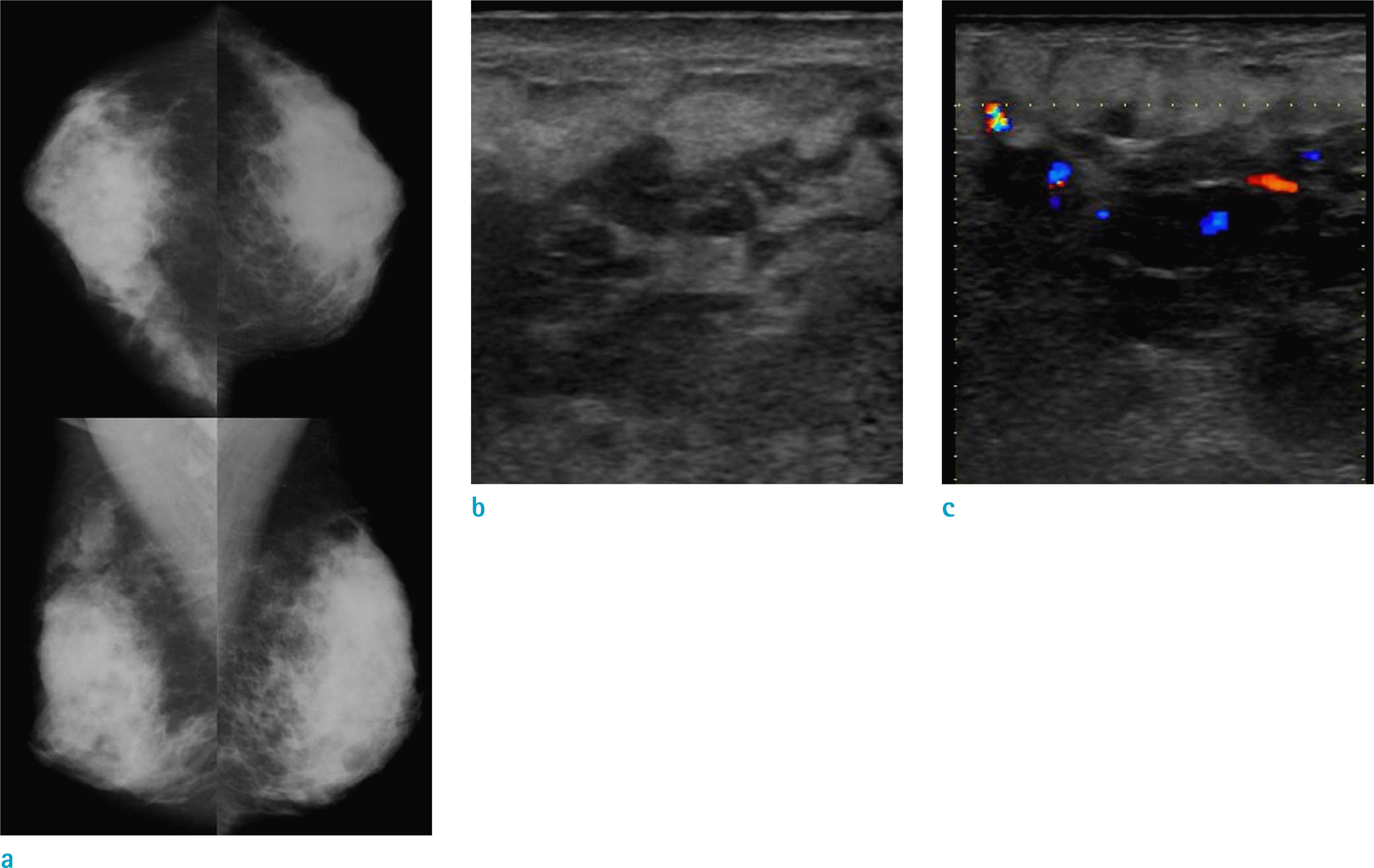
Fig. 2.
MRI shows an irregularly shaped, 4.9 × 4.5 cm sized, low signal mass on T1WI (a) and several, dot-like, high signals (arrows), suggesting internal hemorrhagic foci within a mass. The mass shows high signal intensity on T2WI (b). The central portion of the mass shows early dynamic-phase enhancement (c) and a washout pattern on the delayed phase (d). The periphery of the mass shows persistent trapping of contrast medium in the irregular enhancing portion and surrounding edema. Large, draining vessels (arrowheads) around the mass are well seen on MIP imaging (e). PET-CT (f) shows the inhomogeneous FDG uptake (SUV = 2.2–2.9) of the mass.
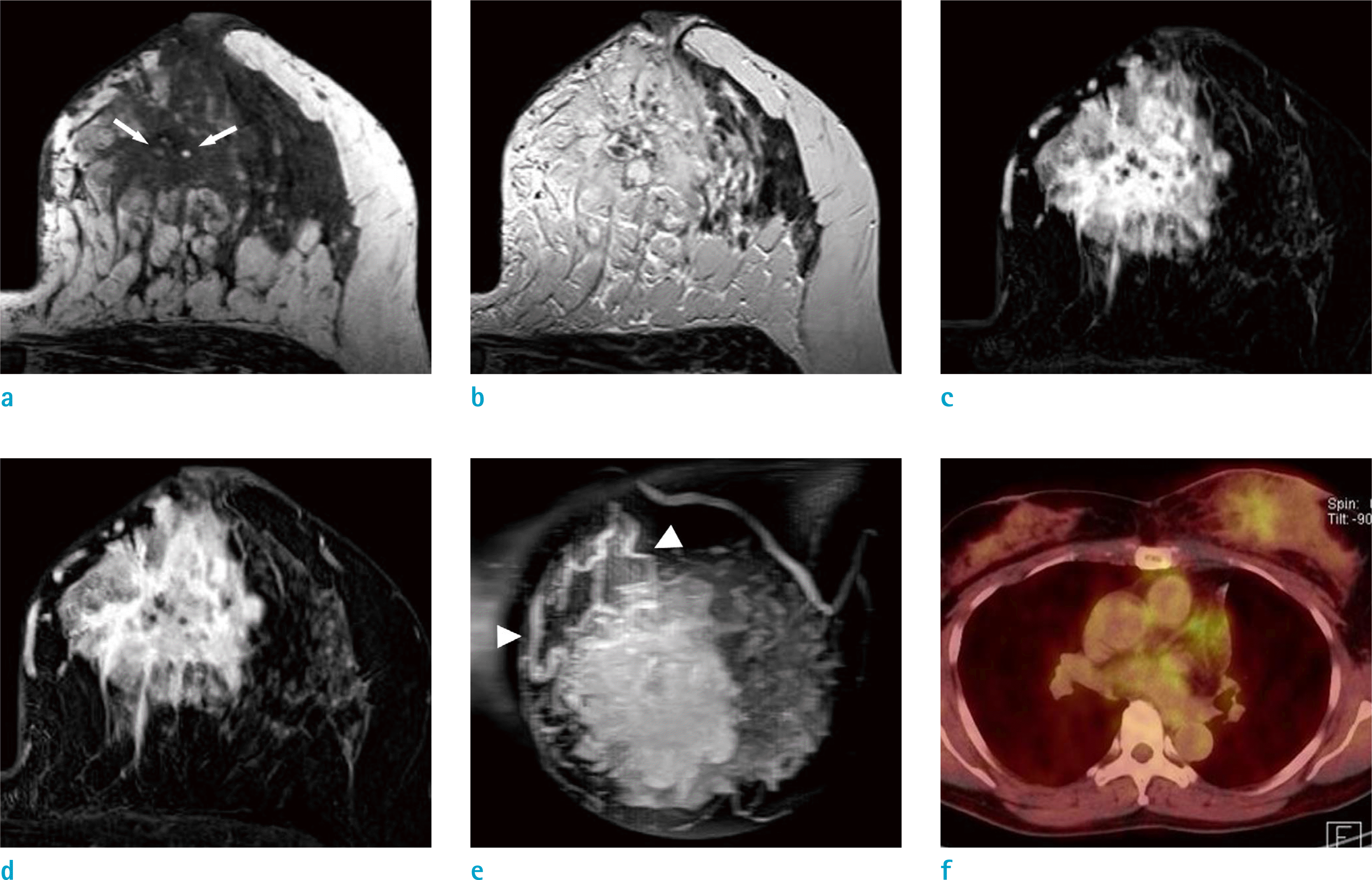
Fig. 3.
A photomicrograph shows the combination of the numerous, inter-anastomosing vascular spaces and more solid, spindle-cell areas effacing the normal lobular architecture (Hematoxylin & Eosin staining, × 200) (a). On immunohistochemical staining, tumor cells are diffusely and strongly positive for CD34 (b) and focally positive for factor VIII (c). The estimated Ki-67 index in the cellular areas is approximately 40% (× 400) (d).

Table 1.
MRI Findings of Primary Breast Angiosarcoma in Articles: the Original MRI Descriptions of Each Article were Used
| Articles | No. of preop. MRI | No. of MRI presented with images | MRI sequences | |||
|---|---|---|---|---|---|---|
| T1 | T2 | Dynamic contrast enhancement | Others sequences/ findings | |||
| Liberman et al. Radiology 1992 (9) | 1 | 1 | Low to intermediate SI (Chest MRI) | SI increased than T1 (Chest MRI) | N/A | Heavily T2 – Tubular areas of the mass, very high SI; vascular channels containing slow flowing blood |
| Marchant et al. AJR Am J Roentgenol 1997 (10) | 2 | 2 | N/A | N/A | N/A | 3D gadolinium enhanced fast spoiled gradient echo Right breast – Enhancing mass with irregular borders. Large draining vessel. Cystic cavity within the mass containing blood. Left breast – Mildly irregular contour enhancing mass. |
| Glazebrook et al. AJR Am J Roentgenol 2005 (14) | 1 | 1 | Increased SI of the area of blood lakes (no image) | N/A | N/A | 3D fast spoiled gradient echo – Multiple nodular areas of rapid and intense contrast enhancement within the mass. Large draining vein |
| Yang et al.* Radiology 2007 (1) | 6 | 2 | Large, lobulated mass Heterogeneously hypointense Irregularly high SI; hemorrhagic nature Markedly heterogeneous architecture | Heterogeneously hyperintense Cystic cavities representing a venous lake | Intense and heterogeneous enhancement with rapid initial enhancement followed by washout kinetic features | Partially irregular and diffuse infiltration Skin thickening Extension to the pectoralis fascia without invasion |
| Kim et al. J Korean Surg Soc 2012 (11) | 1 | 1 | N/A | N/A | Early rapid enhancement with plateau | |
| Dashevsky et al.* Radiol Case Rep 2013 (2) | 1 | 1 | Smooth, lobulated, hypointense mass | Hyperintense | A rapid rise to the peak with washout | STIR – Increased circumferential signal surrounds the mass: edema |
| O'Neill et al. J Med Imaging Radiat Oncol 2014 (15) | 1 | 1 | Low SI (no image) | High SI (no image | e) Irregular, heterogeneous enhancement and plateau kinetics (no Extension to the pectoralis fascia without invasionmage) | MIP – An area of high signal within low signal parenchyma |
| Lin et al.* Medicine 2016 (3) | 1 | 1 | Center – hyperintensity; central intratumoral hematoma Periphery – hypointensity; tumor location | Remarkable inhomogeneous enhancement at the periphery (early) Persist pattern of enhancement (delay) Non-enhancement at the center | Large amount of bridging anastomotic vascular spaces at the tumor periphery; trapping of contrast medium | |
| Iacoponi et al. Int J Surg Case Rep 2016 (16) | 1 | 1 | N/A | N/A | Type 3 pattern | DWI – Solid and hypervascularized components with significant enhancement High ADC Areas with intratumoral bleeding |
| Wang et al.* Breast J 2017 (4) | 13 | 1 | (10/13) Poorly defined lesions with low SI | High SI | Rapid enhancement (early) | A persistent and prolonged enhancement (late) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download