Abstract
Purpose
To investigate the surgical, perfusion, and molecular characteristics of glioblastomas which influence long-term survival after treatment, and to explore the association between MR perfusion parameters and the presence of MGMT methylation and 1p/19q deletions.
Materials and Methods
This retrospective study was approved by our institutional review board. A total 43 patients were included, all with pathologic diagnosis of glioblastoma with known MGMT methylation and 1p/19q deletion statuses. We divided these patients into longterm (≥ 60 months, n = 7) and short-term (< 60 months, n = 36) survivors, then compared surgical extent, molecular status, and rCBV parameters between the two groups using Fisher's exact test or Mann-Whitney test. The rCBV parameters were analyzed according to the presence of MGMT methylation and 1p/19q deletions. We investigated the relationship between the mean rCBV and overall survival using linear correlation. Multivariable linear regression was performed in order to find the variables related to overall survival.
Results
Long-term survivors (100% [7 of 7]) demonstrated a greater percentage of gross total or near total resection than short-term survivors (54.5% [18 of 33]). A higher prevalence of 1p/19q deletions was also noted among the long-term survivors (42.9% [3 of 7]) than the short-term survivors (0.0% [0 of 36]). The rCBV parameters did not differ between the longterm and short-term survivors. The rCBV values were marginally lower in patients with MGMT methylation and 1p/19q deletions. Despite no correlation found between overall survival and rCBV in the whole group, the short-term survivor group showed negative correlation (R2 = 0.181, P = 0.025). Multivariable linear regression revealed that surgical extent and 1p/19q deletions, but not rCBV values, were associated with prolonged overall survival.
References
1. Affronti ML, Heery CR, Herndon JE 2nd, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009; 115:3501–3511.

2. Ostrom QT, Bauchet L, Davis FG, et al. Response to "the epidemiology of glioma in adults: a ‘state of the science’ review". Neuro Oncol. 2015; 17:624–626.

4. Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993; 85:704–710.

6. Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, preclinical development and clinical trials. Cancer Treat Rev. 1997; 23:35–61.

7. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996.

8. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000; 83:588–593.

9. Stupp R, Gander M, Leyvraz S, Newlands E. Current and future developments in the use of temozolomide for the treatment of brain tumours. Lancet Oncol. 2001; 2:552–560.

10. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820.

11. Johnson DR, Guerin JB, Giannini C, Morris JM, Eckel LJ, Kaufmann TJ. 2016 updates to the WHO brain tumor classification system: what the radiologist needs to know. Radiographics. 2017; 37:2164–2180.
12. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018; 44:139–150.

13. Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004; 10:1871–1874.

14. Zhao J, Ma W, Zhao H. Loss of heterozygosity 1p/19q and survival in glioma: a metaanalysis. Neuro Oncol. 2014; 16:103–112.

15. Moon WJ, Choi JW, Roh HG, Lim SD, Koh YC. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology. 2012; 54:555–563.

16. Kim BS, Seol HJ, Nam DH, et al. Concurrent chemoradiotherapy with temozolomide followed by adjuvant temozolomide for newly diagnosed glioblastoma patients: a retrospective multicenter observation study in Korea. Cancer Res Treat. 2017; 49:193–203.

17. Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015; 129:809–827.

18. Blanc JL, Wager M, Guilhot J, et al. Correlation of clinical features and methylation status of MGMT gene promoter in glioblastomas. J Neurooncol. 2004; 68:275–283.

19. Kim SH, Choi SH, Yoon TJ, et al. Measurement of apparent diffusion coefficient values from diffusion-weighted MRI: a comparison of manual and semiautomatic segmentation methods. Investig Magn Reson Imaging. 2015; 19:88–98.

20. Kim YE, Choi SH, Lee ST, et al. Differentiation between glioblastoma and primary central nervous system lymphoma using dynamic susceptibility contrast-enhanced perfusion MR imaging: comparison study of the manual versus semiautomatic segmentation method. Investig Magn Reson Imaging. 2017; 21:9–19.

21. Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994; 191:41–51.

22. Bag AK, Cezayirli PC, Davenport JJ, et al. Survival analysis in patients with newly diagnosed primary glioblastoma multiforme using pre- and posttreatment peritumoral perfusion imaging parameters. J Neurooncol. 2014; 120:361–370.

23. Choi SH, Jung SC, Kim KW, et al. Perfusion MRI as the predictive/prognostic and pharmacodynamic biomarkers in recurrent malignant glioma treated with bevacizumab: a systematic review and a time-to-event metaanalysis. J Neurooncol. 2016; 128:185–194.

24. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and metaanalysis. JAMA Oncol. 2016; 2:1460–1469.
25. Qaddoumi I, Ellison DW, Morris EB, et al. Dysembryoplastic neuroepithelial tumors and cognitive outcome: cure at a price? Cancer. 2010; 116:5461–5469.
26. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: longterm results of RTOG 9402. J Clin Oncol. 2013; 31:337–343.

27. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372:2499–2508.
28. Jenkins RB, Blair H, Ballman KV, et al. A t(1;19) (q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006; 66:9852–9861.
29. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: longterm follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31:344–350.
30. Kaneshiro D, Kobayashi T, Chao ST, Suh J, Prayson RA. Chromosome 1p and 19q deletions in glioblastoma multiforme. Appl Immunohistochem Mol Morphol. 2009; 17:512–516.

31. Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, Kim AH. Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg Focus. 2014; 37:E11.

32. Meng W, Jiang Y, Ma J. Is the prognostic significance of O6-methylguanine-DNA methyltransferase promoter methylation equally important in glioblastomas of patients from different continents? A systematic review with metaanalysis. Cancer Manag Res. 2017; 9:411–425.

33. Stecco A, Amatuzzo P, Sponghini AP, et al. Prognostic value of relative cerebral blood volume (rCBV) in patients with recurrent glioblastoma multiforme treated with bevacizumab. J Neurosurg Sci 2016 [Epub ahead of print].
34. Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008; 247:490–498.

35. Sawlani RN, Raizer J, Horowitz SW, et al. Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging–pilot study. Radiology. 2010; 255:622–628.

36. Cha S, Tihan T, Crawford F, et al. Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2005; 26:266–273.
37. Jenkinson MD, Smith TS, Joyce KA, et al. Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology. 2006; 48:703–713.

38. Bonekamp D, Deike K, Wiestler B, et al. Association of overall survival in patients with newly diagnosed glioblastoma with contrast-enhanced perfusion MRI: comparison of intraindividually matched T1 – and T2 (*)-based bolus techniques. J Magn Reson Imaging. 2015; 42:87–96.
Fig. 2.
Scatter plot of mean rCBV versus overall survival (in months) in GBM patients, including both long-term and short-term survivors. Although the scatter plot demonstrates a decreasing trend towards lower mean rCBV values with greater overall survival, the linear regression analysis did not demonstrate a statistically significant linear correlation (P = 0.186, R2 = 0.029).
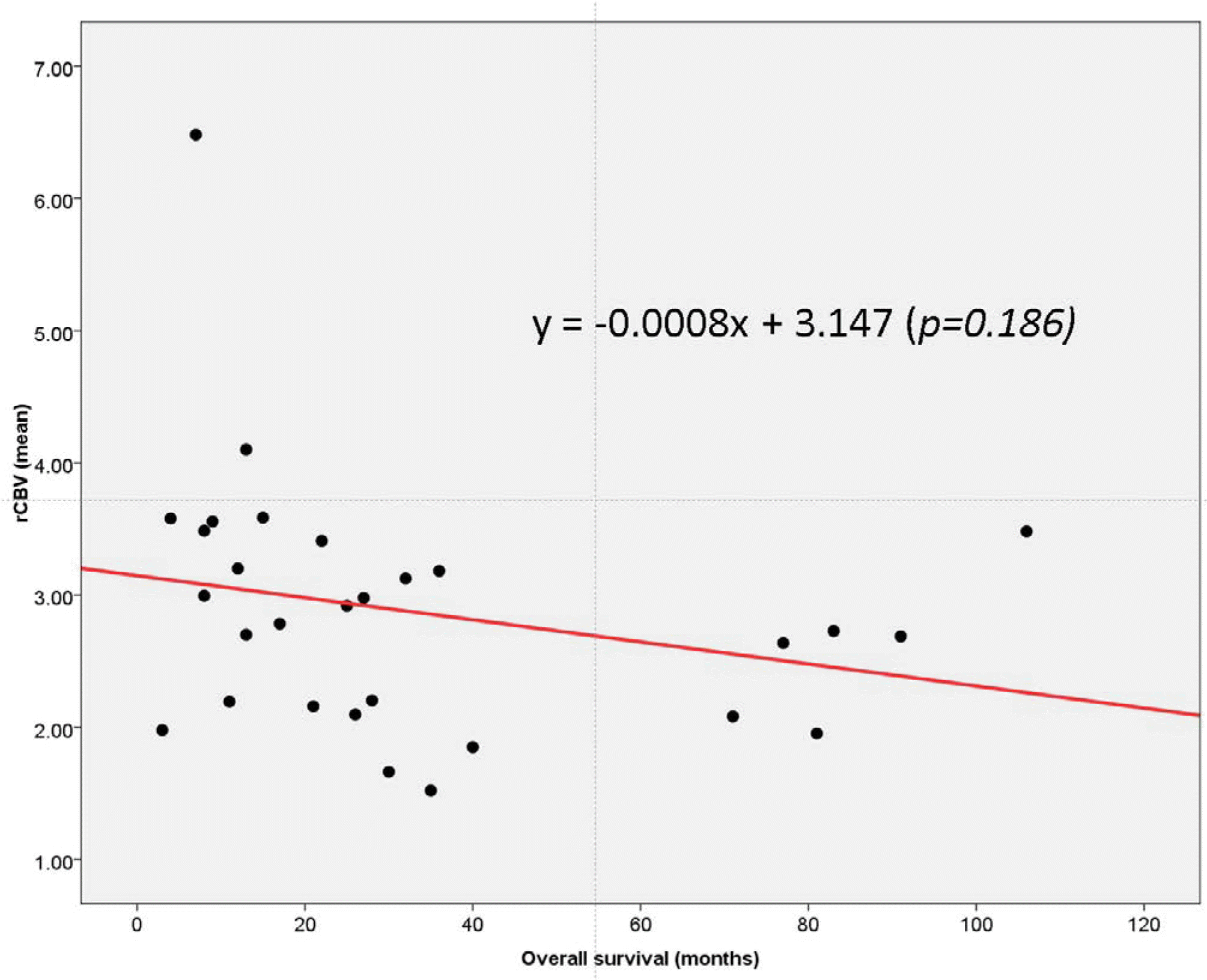
Fig. 3.
Scatter plot of mean rCBV versus overall survival (in months) in short-term GBM survivors. Linear regression analysis demonstrated the presence of a statistically significant negative linear correlation between mean rCBV and overall survival (P = 0.025, R2 = 0.181).
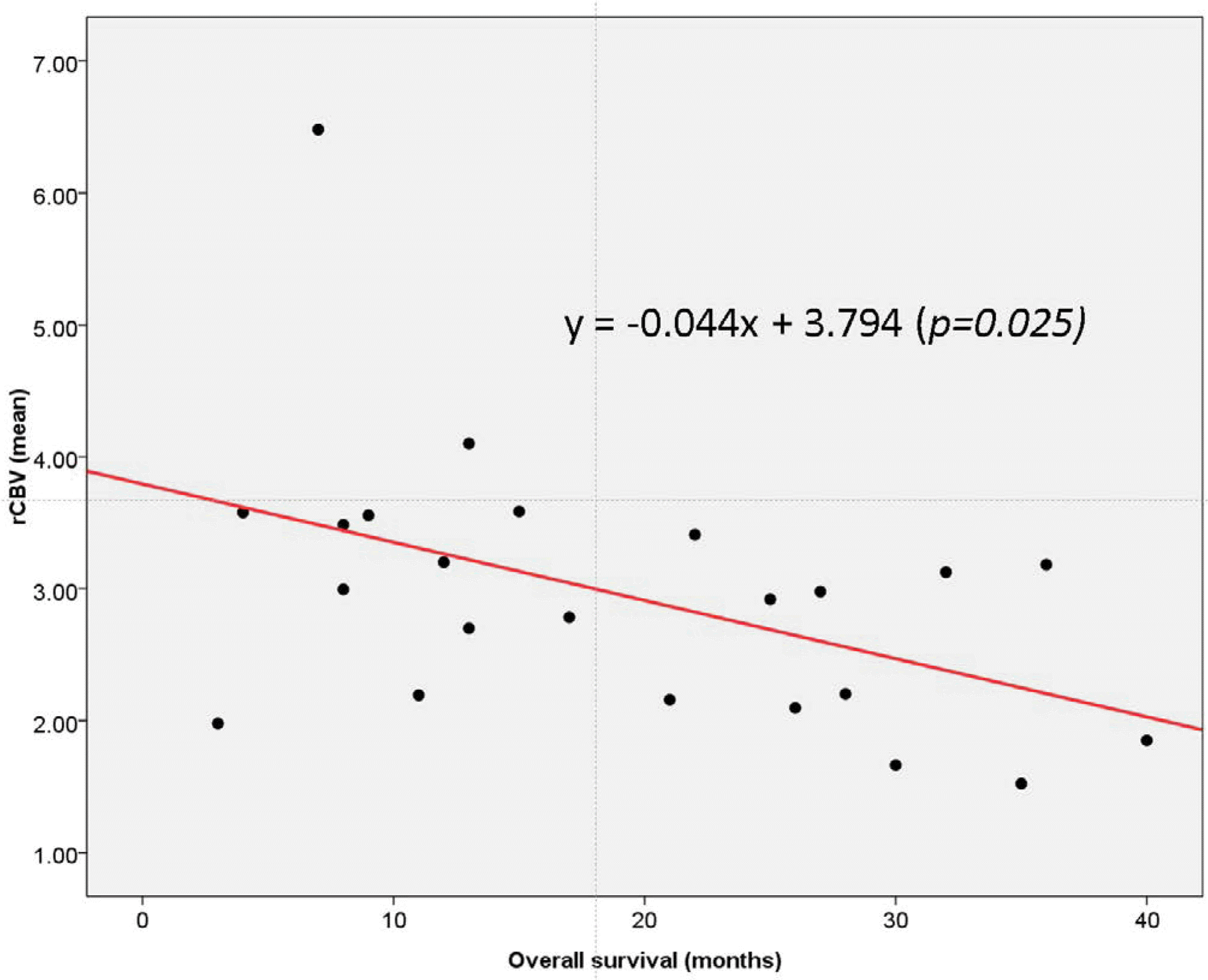
Fig. 4.
A 47-year-old male with glioblastoma in the right frontal lobe. (a) T2-weighted image, (b) T1-weighted contrast-enhanced image, and (c) rCBV map are shown. The mean rCBV value measured from the enhanced part of the tumor was 1.95. Molecular analysis revealed both MGMT promoter methylation and 1p19q codeletion. The overall survival was 81 months.
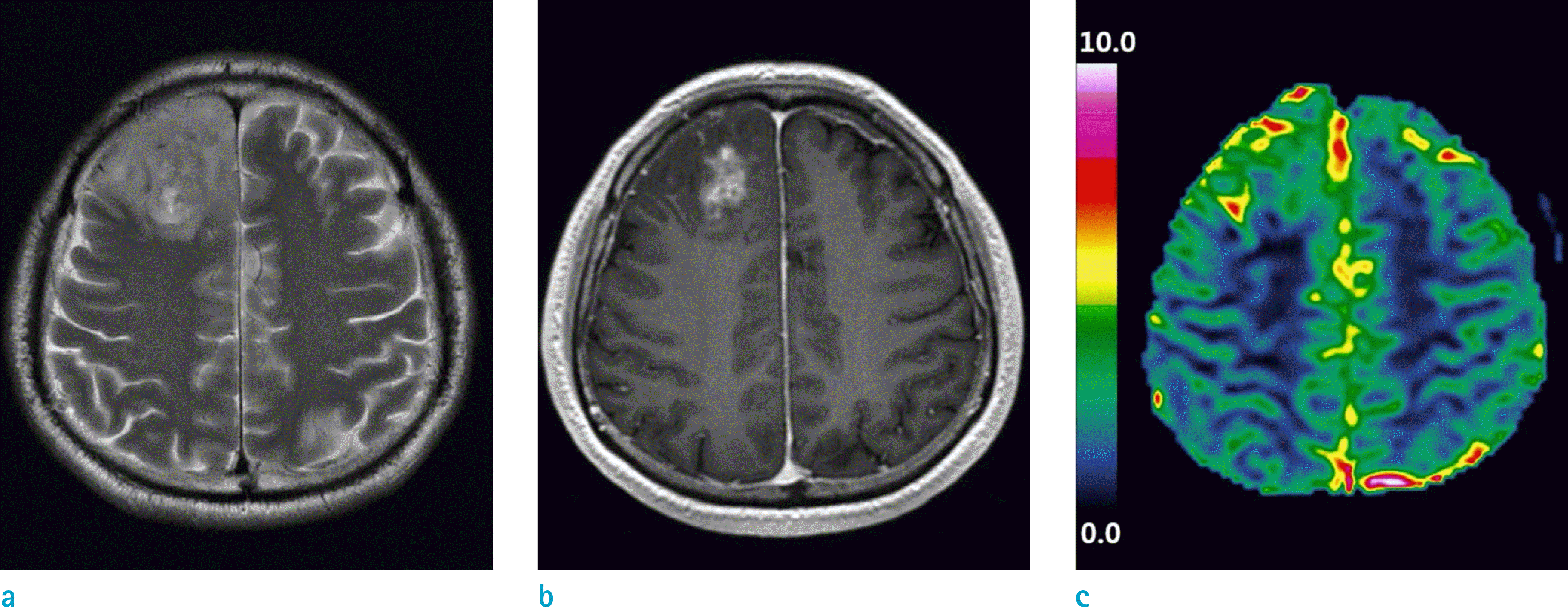
Fig. 5.
A 73-year-old male with glioblastoma in the left temporal lobe. (a) T2-weighted image, (b) T1-weighted contrast-enhanced image, and (c) rCBV map are shown. The mean rCBV value measured from the enhanced part of the tumor was 2.70. Molecular analysis revealed that both MGMT promoter and 1p19q were intact. The overall survival was 13 months.
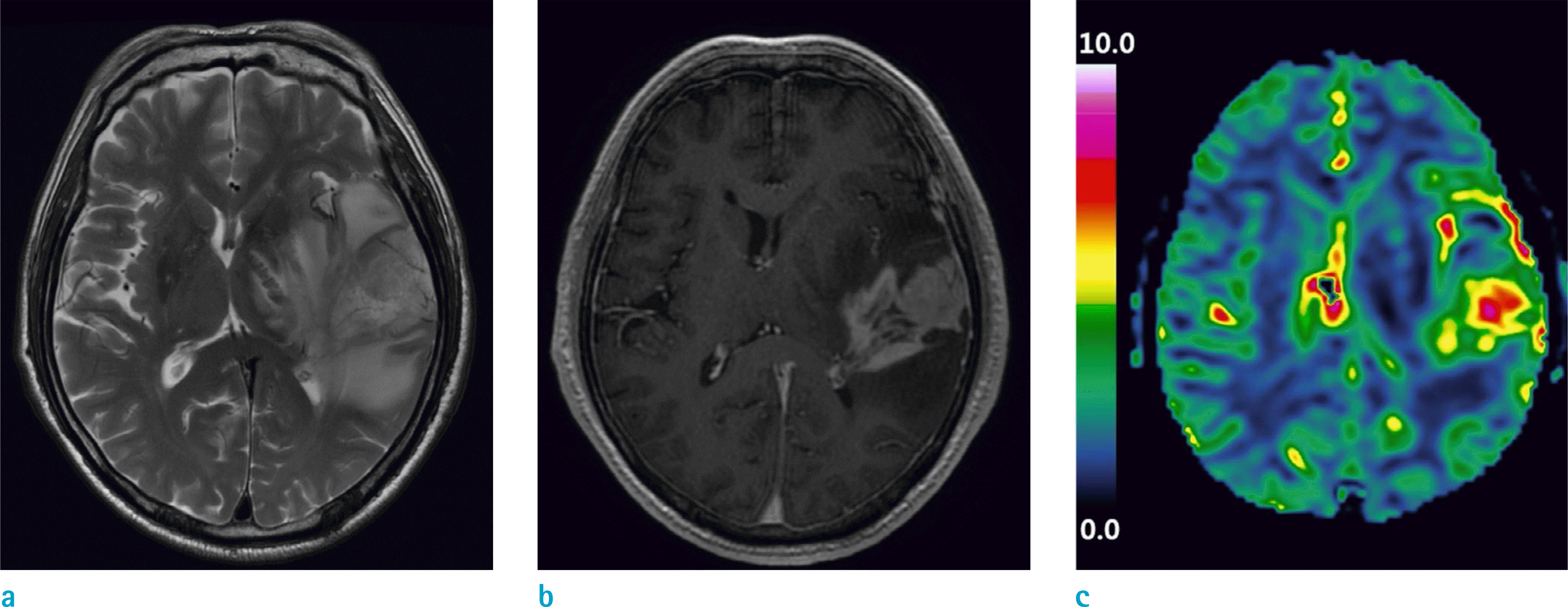
Fig. 6.
A 43-year-old female with glioblastoma in the right frontal lobe. (a) T2-weighted image, (b) T1-weighted contrast-enhanced image, and (c) rCBV map are shown. The mean rCBV value measured from the enhanced part of the tumor was 3.48. The molecular analysis revealed that both MGMT promoter and 1p19q were intact. The overall survival was 106 months.
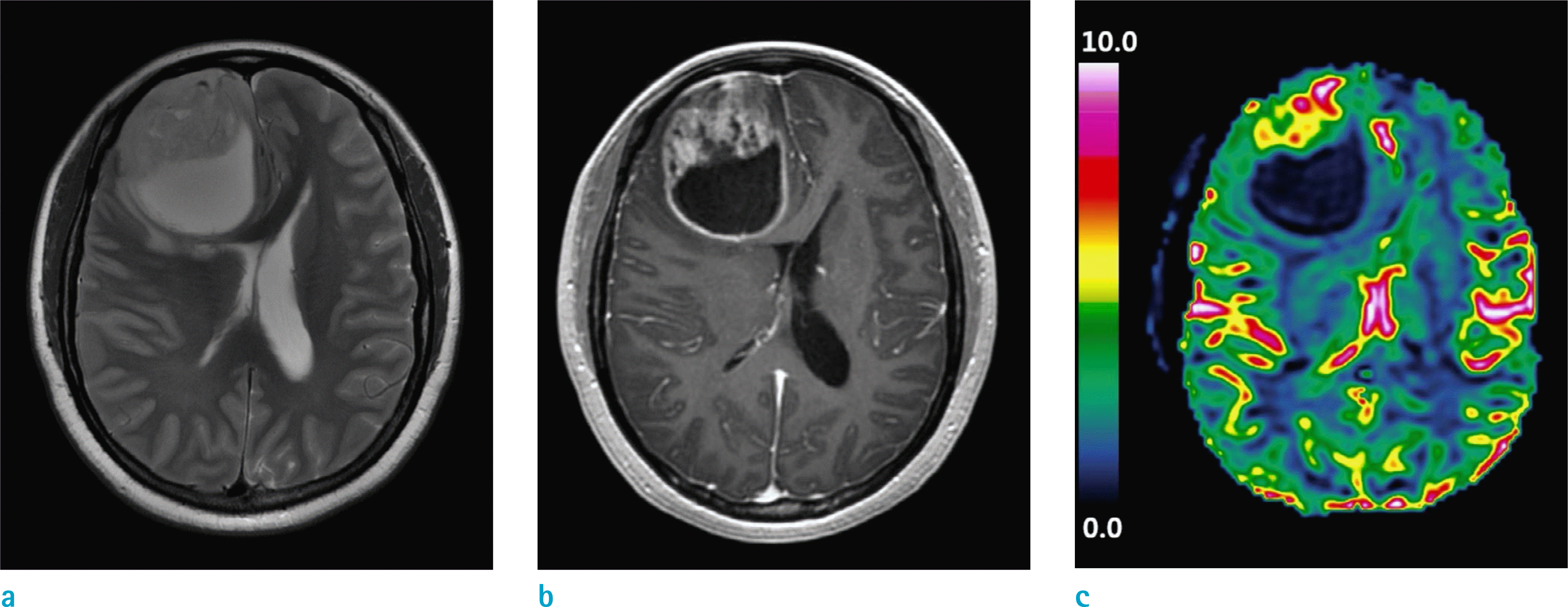
Table 1.
Demographic Characteristics of the Study Subjects
Data are number of patients, and data in parenthesis are percentages except where indicated. *The data is the mean value and the data in parenthesis is range of the values. ** Abbreviations area indicated as follows: GTR = gross total resection; NTR = near total resection; STR = subtotal resection; PR = partial resection; Bx = biopsy *** 40 of 43 patients were evaluated for surgical extent. Three patients who could not undergo immediate postoperative (< 24 hours) MRI were excluded because the surgical extent could not be accurately measured.
Table 2.
Comparison of Various rCBV Parameters between the Long-Term and Short-Term Survivors
Table 3.
Comparison of Various rCBV Parameters in Terms of MGMT Methylation and 1p/19q Deletions
Table 4.
Multivariate Analysis of Predicting Overall Survival in Terms of Surgical Extent, 1p/19q Deletion, MGMT Methylation and Mean rCBV
| Variables | Adjusted R2 | P-value, univariate | P-value, multivariate |
|---|---|---|---|
| Surgical extent | 0.163 | 0.017 | 0.042 |
| 1p/19q deletions | 0.188 | 0.011 | 0.039 |
| MGMT methylation | –0.035 | 0.826 | 0.490 |
| Mean rCBV | 0.029 | 0.186 | 0.273 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download