Abstract
Purpose
To assess associations between morphological characteristics of intracranial arteries in time-of-flight MR angiography (TOF-MRA) and atherosclerotic risk factors.
Materials and Methods
From January 2014 to October 2015, a total of 129 patients (65 men and 64 women) without intracranial arterial stenosis > 50% were included in this study. All MRIs were performed using a 3T machine with 3D TOF-MRA sequences. We evaluated irregularity, tortuosity, and dilatation of intracranial arteries in maximal intensity projection (MIP) of TOF-MRA. Subjects’ risk factors for atherosclerosis including history of hypertension and diabetes were collected by reviewing their medical records. Associations between morphological characteristics and each known atherosclerosis risk factor were examined using univariate regression analysis. Multivariate regression models were built to determine combined association between those risk factors and morphologic changes of intracranial arteries.
Results
In multivariate analysis, hypertension (coefficient [95% CI]: 0.162 [0.036, 0.289], P = 0.012) and absence of diabetes (coefficient [95% CI]: −0.159 [−0.296, −0.023], P = 0.022) were associated with large diameter of intracranial arteries. Males (coefficient [95% CI]: 0.11 [−0.006, 0.23], P = 0.062) and higher age (coefficient [95% CI]: 0.003 [−0.001, 0.008], P = 0.138) had marginal association with increased diameter. Tortuosity was associated with old age (OR: 1.04 [1.02, 1.07], P < 0.001). Irregular contour of intracranial arteries was significantly associated with old age (OR: 1.05 [1.02, 1.09], P = 0.004), presence of diabetes (OR: 2.88 [1.36, 6.15], P = 0.0058), and previous ischemic stroke (OR: 3.91 [1.41, 11.16], P = 0.0092).
References
1. Li MH, Li YD, Gu BX, et al. Accurate diagnosis of small cerebral aneurysms </=5 mm in diameter with 3.0-T MR angiography. Radiology. 2014; 271:553–560.
2. Korogi Y, Takahashi M, Mabuchi N, et al. Intracranial vascular stenosis and occlusion: diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology. 1994; 193:187–193.

3. Lee WJ, Choi HS, Jang J, et al. Non-stenotic intracranial arteries have atherosclerotic changes in acute ischemic stroke patients: a 3T MRI study. Neuroradiology. 2015; 57:1007–1013.

4. Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis: a 3-tesla MRI study. Neurology. 2010; 75:253–258.

5. Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke. 2016; 47:434–440.

6. Degnan AJ, Gallagher G, Teng Z, Lu J, Liu Q, Gillard JH. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR Am J Neuroradiol. 2012; 33:1427–1435.

7. Pico F, Labreuche J, Touboul PJ, Leys D, Amarenco P. Intracranial arterial dolichoectasia and small-vessel disease in stroke patients. Ann Neurol. 2005; 57:472–479.

8. Gutierrez J, Sacco RL, Wright CB. Dolichoectasia-an evolving arterial disease. Nat Rev Neurol. 2011; 7:41–50.

9. Han J, Qiao H, Li X, et al. The three-dimensional shape analysis of the M1 segment of the middle cerebral artery using MRA at 3T. Neuroradiology. 2014; 56:995–1005.

10. Hoi Y, Gao L, Tremmel M, et al. In vivo assessment of rapid cerebrovascular morphological adaptation following acute blood flow increase. J Neurosurg. 2008; 109:1141–1147.

11. Bullitt E, Gerig G, Pizer SM, Lin W, Aylward SR. Measuring tortuosity of the intracerebral vasculature from MRA images. IEEE Trans Med Imaging. 2003; 22:1163–1171.

12. Diedrich KT, Roberts JA, Schmidt RH, Kang CK, Cho ZH, Parker DL. Validation of an arterial tortuosity measure with application to hypertension collection of clinical hypertensive patients. BMC Bioinformatics. 2011; 12(Suppl 10):S15.

13. Lee SJ, Kim JS, Chung SW, Kim BS, Ahn KJ, Lee KS. White matter hyperintensities (WMH) are associated with intracranial atherosclerosis rather than extracranial atherosclerosis. Arch Gerontol Geriatr. 2011; 53:e129–132.

14. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 9:689–701.

15. Park JH, Kwon HM, Lee J, Kim DS, Ovbiagele B. Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur J Neurol. 2015; 22:44–52. e42–43.

16. Lee SJ, Kim JS, Lee KS, et al. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. 2008; 8:31.

17. Dieleman N, Yang W, Abrigo JM, et al. Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis. Stroke. 2016; 47:1797–1802.

18. Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis. 2009; 27:433–442.

19. Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006; 113:555–563.

20. Tsuruda J, Saloner D, Norman D. Artifacts associated with MR neuroangiography. AJNR Am J Neuroradiol. 1992; 13:1411–1422.
21. Fazekas F, Barkhof F, Wahlund LO, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002; 13(Suppl 2):31–36.

22. Saffari SE, Love A, Fredrikson M, Smedby O. Regression models for analyzing radiological visual grading studies–an empirical comparison. BMC Med Imaging. 2015; 15:49.

23. Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008; 39:1142–1147.

24. Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012; 135:3749–3756.

25. Klein IF, Lavallee PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010; 41:1405–1409.
26. Del Corso L, Moruzzo D, Conte B, et al. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998; 49:361–371.

27. Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000; 248:7–12.
28. Dobrin PB, Schwarcz TH, Baker WH. Mechanisms of arterial and aneurysmal tortuosity. Surgery. 1988; 104:568–571.
29. Hegedus K. Ectasia of the basilar artery with special reference to possible pathogenesis. Surg Neurol. 1985; 24:463–469.
31. Charidimou A, Martinez-Ramirez S, Reijmer YD, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: An imaging-pathologic study of concept validation. JAMA Neurol. 2016; 73:994–1001.
Fig. 1.
Patient selection flow chart. After systematic review of radiologic database, we included 129 subjects in this study.
Fig. 2.
A scheme showing seven segments of assessed intracranial arteries. Blue = basilar arteries; Green = distal internal carotid arteries; Orange = V4 segments of vertebral arteries; Yellow = M1 segments of middle cerebral arteries
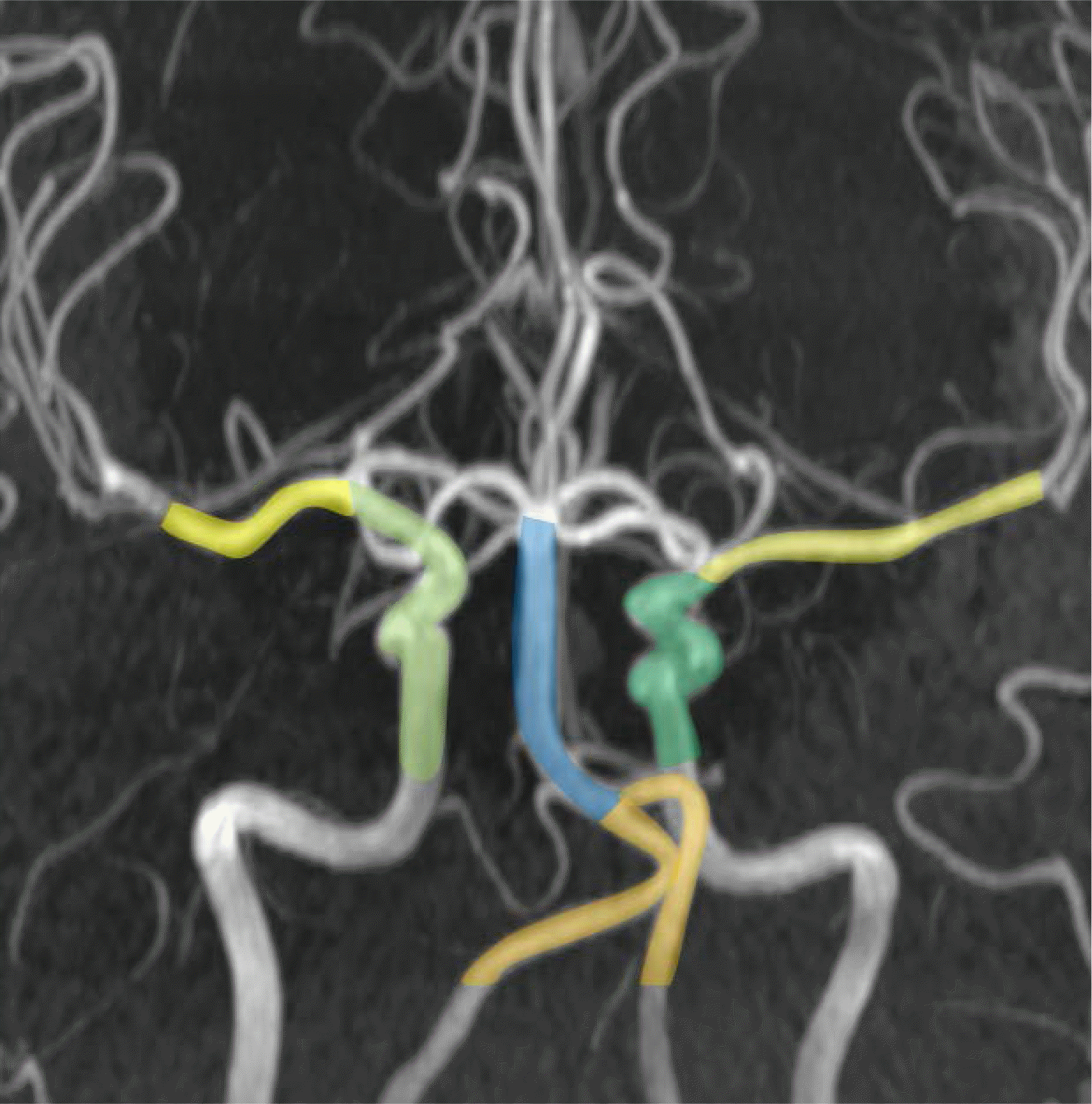
Fig. 3.
Three schemes used to assess morphological features of intracranial arteries. Each panel explains criteria for irregularity (a), tortuosity (b), and diameter (c).
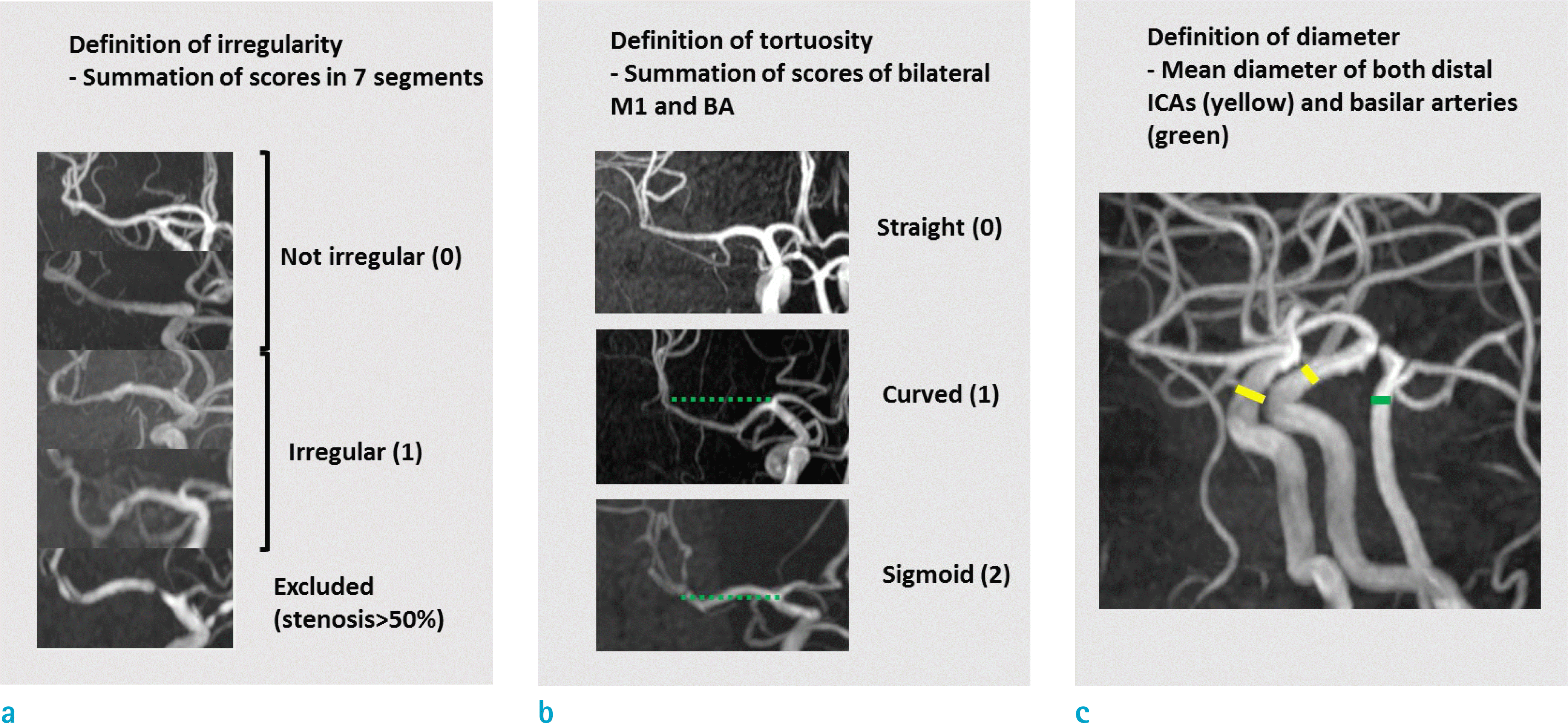
Table 1.
Patients Characteristics (n = 129)
Table 2.
Univariate Analysis Results
Table 3.
Multivariate Analysis Results




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download