This article has been
cited by other articles in ScienceCentral.
Abstract
We report a case of bilateral perisylvian polymicrogyria, which was evaluated using diffusion tensor imaging (DTI) and tractography. On DTI tractography, fibers of the arcuate fasciculus (AF), which connects the posterior inferior frontal region and superior temporal gyrus were absent. It indicates that in cases of bilateral perisylvian polymicrogyria, compromised language skills might be associated with the absence of AF.
Highlights
• Mild mental retardation (IQ 43 and SQ 58.25) was documented on Korean-Wechsler Intelligence Scale for Children-third edition and Social Maturation Scale.
• However, receptive language scale (71.4) was better than expressive one (35.71) documented by Preschool Receptive-Expressive Language scale test.
Keywords: Diffusion Tensor Imaging, Polymicrogyria, Language
INTRODUCTION
Congenital bilateral perisylvian polymicrogyria occurs due to bilateral perisylvian cortical malformations, and is characterized by pseudobulbar palsy, feeding disturbances and facial diparesis as well as seizures since early infancy [
12345]. The speech impairment was more pronounced than what would be expected from the severity of the cortical pseudobulbar palsy. Thus, it has been suggested that the linguistic defect in congenital bilateral perisylvian polymicrogyria patients was caused due to the dysfunction of the perisylvian language networks of the brain [
67].
However, it has been explored yet in detail about the functional abnormalities of the perisylvian language networks in these patients. Diffusion tensor imaging (DTI), coupled with tractography, offers a non-invasive technique that reconstructs the white matter trajectories in the living human brain, and provides a more accurate identification of the white matter dysgenesis and injury, than the conventional magnetic resonance imaging (MRI) does [
8].
In the current study, we used diffuse tensor tractography (DTT) to identify the dysfunction of the perisylvian language networks. We focused our analysis on the arcuate fasciculus (AF), which belongs to the core perisylvian circuit underlying the speech function.
We report here, the case of 10-year old girl with congenital bilateral perisylvian polymicrogyria with absence of arcuate fascicle in bilateral hemispheres, detected using DTT.
CASE REPORT
The patient was a 10-year-old female. She visited our institution for evaluation of severe dysarthria. Gestation and pregnancies were unremarkable. She had delayed developmental milestones with severe delay in developing speech. She had difficulty in sucking during the perinatal period. Family history revealed no evidence of consanguinity and no familial occurrence of the disease. She had infantile spasms during the first year of life, and developed partial seizures at the age of 10 years. The seizures were sporadic in frequency. She was predominantly left-handed. Manual muscle testing revealed a muscle strength grade of 5/5 in the upper and lower limbs with an exception of the left ankle dorsiflexor, which had a strength of 3/5. Five to six clonic contractions were seen at the left ankle. She was able to walk independently from the age of 24 months. Development of language skills was severely impaired. She had a marked tongue movements deficit, with severe dysarthria and a nasal tone. The lingual and labial sounds were significantly affected. She also showed oromotor apraxia and pseudobulbar symptoms. Mild mental retardation (IQ 43 and SQ 58.25) was documented on the Korean-Wechsler Intelligence Scale for Children-third edition and Social Maturation Scale. Given the degree of mental impairment, aural comprehension was normal for words and short phrases, but she showed impaired comprehension for complex, sentence-length spoken words. However, her receptive language scale (71.4) was better than the expressive scale (35.71), documented by Preschool Receptive-Expressive Language Scale test.
Magnetic resonance images (MRIs) revealed an abnormally thickened frontoparietal perisylvian cortex bilaterally, which was clearly delineated from the underlying white matter and consistent with bilateral parietal polymicrogyria (
Fig. 1). In order to explore the speech abnormalities associated with bilateral perisylvian microgyria, we performed a diffusion tensor MRI and tractography, and compared the results with an age-matched normal control.
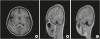 | Fig. 1 T1-weighted axial (A) and parasagittal (B, left; C, right) magnetic resonance images show thick cortex and multiple small gyri and sulci in the bilateral perisylvian areas of patient.
|
Diffusion tensor images were obtained with a head coil on a 3.0T Siemens Verio scanner (Siemens, Erlangen, Germany) using a single-shot echo planar imaging sequence with two diffusion sensitizing gradients. To reduce the duration of the scan, the Generalized Autocalibrating Partially Parallel Acquisition (GRAPPA) technique, which is a part of the parallel imaging technique was used. Moreover, this technique produces better image quality because of the reduction in image distortion caused by the echo-planar imaging sequence. For correcting the potential image distortion, an automated image registration program was employed. The imaging parameters used were: echo time = 108 ms, repetition time = 7,000 ms, field of view = 200 mm2, matrix size = 128 × 128, number of excitations = 2, and b-value = 1,000 s/mm2. We acquired 46 contiguous slices of 3.0 mm slices, parallel to the anterior commissure-posterior commissure line with no gap in 30 different diffusion directions.
Fiber tracking was processed using DTI Studio (Johns Hopkins University, Baltimore, MD, USA;
http://cmrm.med.jhmi.edu) based on fiber assignment using the continuous tracking algorithm which can estimate a single dominant diffusion orientation within the imaging voxel [
9]. The termination criteria used for fiber tracking were fractional anisotropy (FA) < 0.25, angle < 70˚. The seed region of interest was manually selected in each hemisphere on a coronal slice, on which the arcuate fascicle appearing as a green triangular shape-was seen to be the largest. A target region was used on an axial slice through which the arcuate fascicle passes in the inferior/superior direction [
10].
The frontoparietal portion of the arcuate fascicle encompasses a group of fibers in the antero-posterior direction, running lateral to the projection fibers of the corona radiata in axial color maps of a normal child used as control. The axial FA color map regions corresponding to the arcuate fascicle showed green fibers lateral to the vertical corona radiata in the control subject (
Fig. 2B and D). However, the bilateral arcuate fascicles were not seen in the patient (
Fig. 2A and C). In addition, DTT of both hemispheres also demonstrated that the arcuate fascicle, which is a lateral associated bundle composed of long and short fibers connecting the perisylvian cortex of the frontal, parietal, and temporal lobes was not delineated (
Fig. 3A and B). The fibers of the arcuate fascicle from the postero-inferior frontal region descended into the superior temporal gyrus in the child used as a control subject (
Fig. 3C and D).
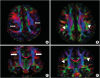 | Fig. 2
The axial color map shows the disorganized whiter matter and absence of the bilateral AF resulting in lack of green (arrow) in a patient with bilateral perisylvian polymicrogyria (A, C). In control, AF is located lateral to the corona radiata and appearing of AF as a green (indicating anterior-posterior orientation) triangular shape was seen in each hemisphere (arrow head) (B, D).
AF, arcuate fasciculus.

|
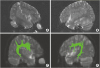 | Fig. 3
The results of fiber tracking in the patient (A, left; B, right) and control (C, left; D, right). The fibers from the posterior inferior frontal region do not descend into the superior temporal gyrus in the developmentally delayed child demonstrating the absence of classic AF in the patient (A, B). The fibers of AF from posterior inferior frontal region descend into superior temporal gyrus in the typically developing child (C, D).
AF, arcuate fasciculus.

|
DISCUSSION
This would be the first case report, which showed the absence of arcuate fascicle in a patient with congenital bilateral perisylvian polymicrogyria.
It is important to note that the reconstructed tracts were not actual white matter fibers, but represented streamlines based on mathematically derived tensor directions. Since the fiber tracts cannot be seen at a microscopic level, DTT is the only technique that allows the identification of the white matter pathways in the living human brain. The exact location and extent of perisylvain language pathways can be identified noninvasively in vivo by means of DTI.
The arcuate fascicle is a major white matter tract that is one of the primary fiber bundles involved in human language processing. This tract connects the Broca's area in the frontal lobe, a region mainly involved in speech production, with the Wernicke's area in the temporal lobe, a region related to speech comprehension [
11].
The absence of AF in our patient might have been caused due to the failure of formation of appropriate connections between the Wernicke's area and the Broca's area [
11]. Abnormalities in the axonal target neurons or abnormalities in molecular pathways involved in the axonal path findings around the sylvian fissure are the possible underlying mechanisms for the absence of AF in our patient [
12]. The correct wiring of the nervous system relies on the axonal guidance, which navigate over long distances along the specific pathways to find their correct targets [
13]. Further mechanistic studies are required to explore the mechanisms for the absence of AF in a patient with congenital bilateral perisylvian polymicrogyria.
In our patient, a severely impaired speech function was accompanied by oromotor dyspraxia followed by impaired tongue movement and swallowing. This indicates that the absence of the AF seems to be associated with dyspraxia and that AF might be involved in auditory-motor integration by mapping the acoustic sounds to articulatory representation [
314].
Our findings provide a mechanism for understanding impaired speech in a patient with congenital bilateral perisylvian polymicrogyria. This study is limited because it is a case report. Further research is necessary by imaging children with congenital bilateral perisylvian polymicrogyria.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download