1. Ishido M, Horita N, Takeuchi M, Shibuya E, Yamane T, Kawagoe T, Ishido T, Minegishi K, Yoshimi R, Kirino Y, Hirohata S, Ishigatsubo Y, Takeno M, Kaneko T, Mizuki N. Distinct clinical features between acute and chronic progressive parenchymal neuro-Behçet disease: meta-analysis. Sci Rep. 2017; 7:10196.

2. Yoon DL, Kim YJ, Koo BS, Kim YG, Lee CK, Yoo B. Neuro-Behçet's disease in South Korea: clinical characteristics and treatment response. Int J Rheum Dis. 2014; 17:453–458.

3. Hirohata S, Kikuchi H, Sawada T, Nagafuchi H, Kuwana M, Takeno M, Ishigatsubo Y. Clinical characteristics of neuro-Behcet's disease in Japan: a multicenter retrospective analysis. Mod Rheumatol. 2012; 22:405–413.

4. Fisher CA, Sewell K, Baker A. Chronic behavior disturbance and neurocognitive deficits in neuro-Behcet's disease: a case study. Neurocase. 2016; 22:332–338.

5. Cavaco S, da Silva AM, Pinto P, Coutinho E, Santos E, Bettencourt A, Pinto C, Gonçalves A, Silva S, Gomes F, Carvalho L, Pereira C, Martins B, Correia J, Vasconcelos C. Cognitive functioning in Behçet's disease. Ann N Y Acad Sci. 2009; 1173:217–226.

6. Mimura M, Kato M, Kashima H. Neuro-Behcet's disease presenting with amnesia and frontal dysfunction. Clin Neurol Neurosurg. 2009; 111:889–892.

7. van Ham C, Schrijvers D, De Picker L, Vandendriessche F, Sabbe B. Neuropsychiatric features in Behçet's disease: a case report. Clin Neurol Neurosurg. 2014; 127:13–14.

8. Peño IC, De las Heras Revilla V, Carbonell BP, Di Capua Sacoto D, Ferrer ME, García-Cobos R, González RA. Neurobehçet disease: clinical and demographic characteristics. Eur J Neurol. 2012; 19:1224–1227.

9. International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990; 335:1078–1080.
10. Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, Houman H, Mahr A, Salvarani C, Sfikakis PP, Siva A, Al-Araji A. Diagnosis and management of Neuro-Behçet's disease: international consensus recommendations. J Neurol. 2014; 261:1662–1676.

11. Kim J, Oh BM, Kim JY, Lee GJ, Lee SA, Han TR. Validation of the videofluoroscopic dysphagia scale in various etiologies. Dysphagia. 2014; 29:438–443.

12. Kang Y, Jahng S, Na DL. Seoul neuropsychological screening battery, 2nd edition (SNSB-II). Seoul: Human Brain Research & Consulting Co.;2012.
13. Al-Araji A, Kidd DP. Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurol. 2009; 8:192–204.

14. de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D. Thalamic control of human attention driven by memory and learning. Curr Biol. 2014; 24:993–999.
15. Noel N, Bernard R, Wechsler B, Resche-Rigon M, Depaz R, Le Thi Huong Boutin D, Piette JC, Drier A, Dormont D, Cacoub P, Saadoun D. Long-term outcome of neuro-Behçet's disease. Arthritis Rheumatol. 2014; 66:1306–1314.

16. Duman I, Guzelkucuk U, Tezel K, Aydemir K, Yılmaz B. Behcet's disease presenting with sudden-onset paraplegia due to anterior spinal artery involvement: 1-year follow-up of rehabilitation in conjunction with medication. Rheumatol Int. 2013; 33:1605–1608.

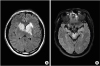





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download