Abstract
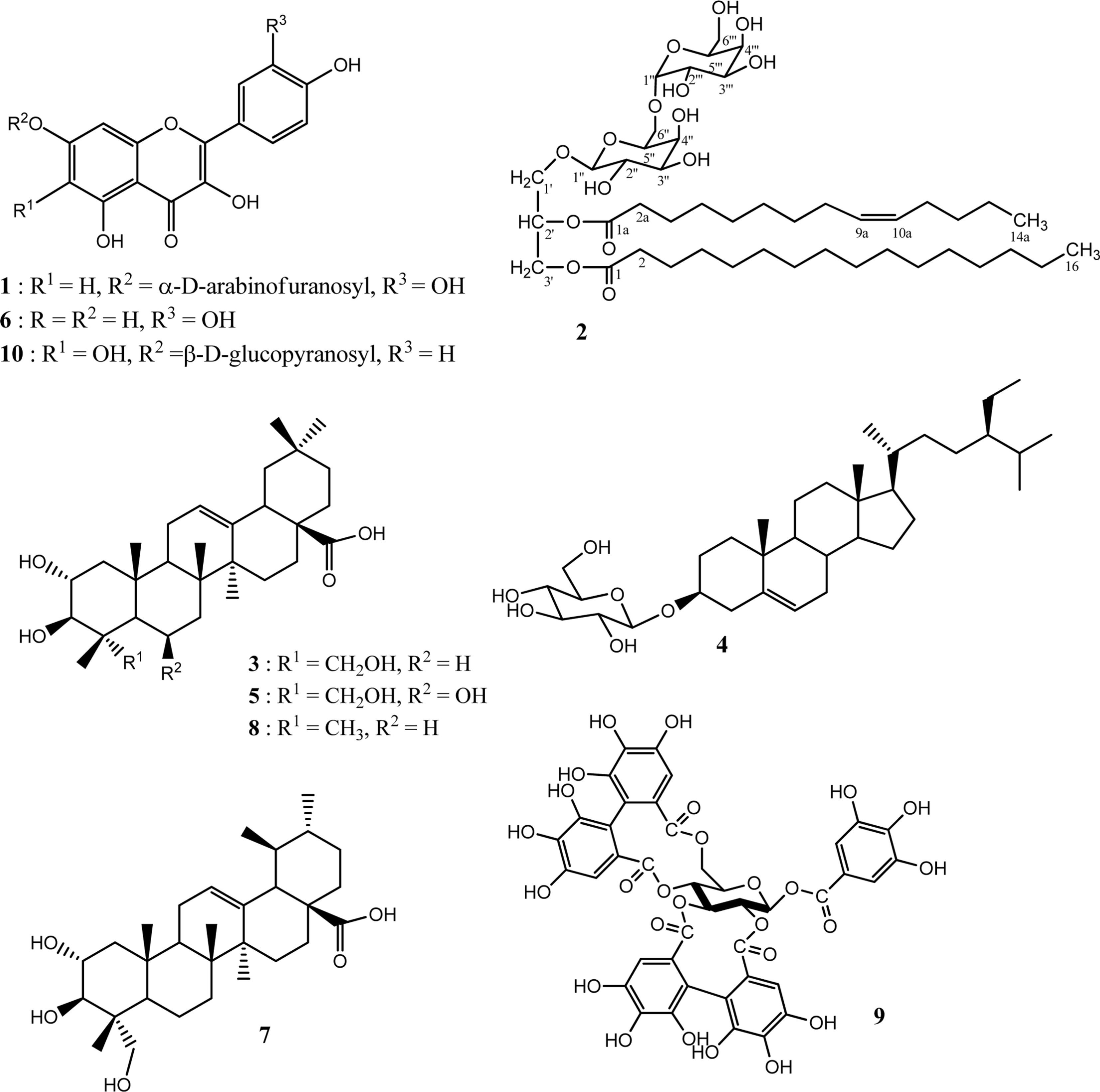
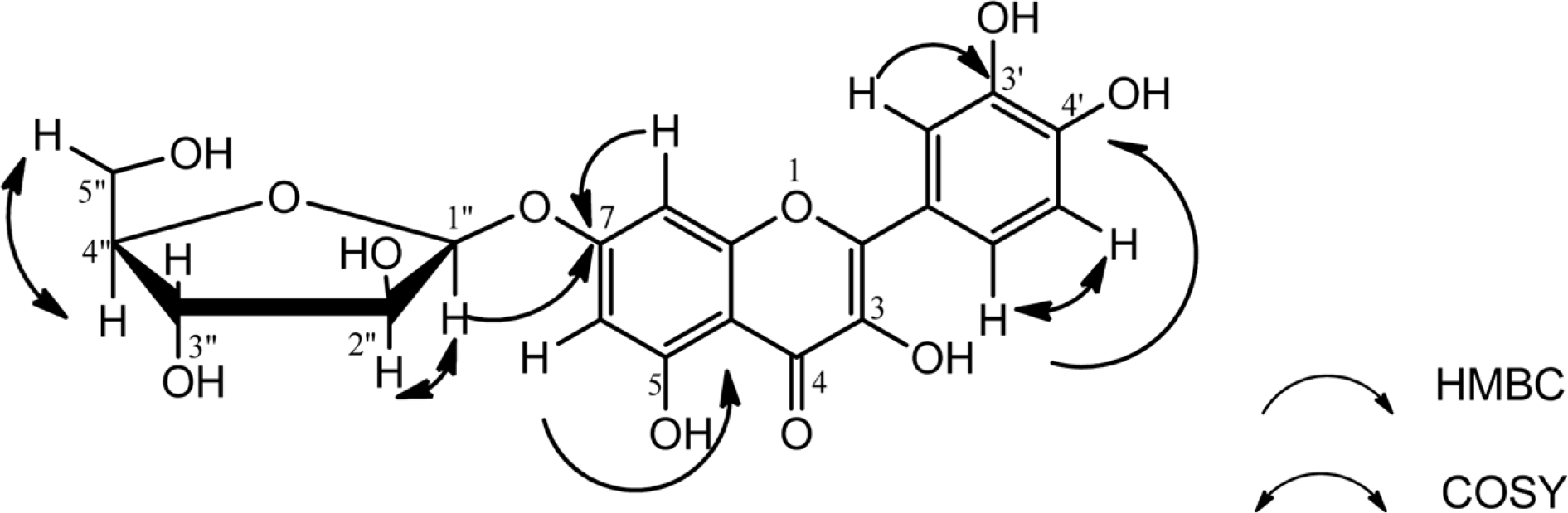
Chemical investigation of the plant Tristemma hirtum P. Beauv (Melastomataceae) resulted to the isolation of a new flavonol glycoside named quercetin-7-O-α-D-arabinofuranoside (1), together with nine known compounds including 3'-hexadecanoyl-2'-(9aZ)-tetradecanoyl-glycerol 1'-O-[β-D-galactopyranosyl-(1″ → 6″)-α-D-galactopyranoside] (2), arjunolic acid (3), β-sitosterol-3-O-β-D-glucopyranoside (4), terminolic acid (5), quercetin (6), asiatic acid (7), maslinic acid (8), 1β-O-galloylpedunculagin (9) and 6-hydroxyapigenin 7-O-β-D-glucopyranoside (10) from the methanol extract using normal and reversed phase column chromatography. The structures of these compounds were determined by comprehensive interpretation of their spectral data mainly including 1D- 2D-NMR (1H-1H COSY, HSQC, and HMBC) spectroscopic and ESI-TOF-MS mass spectro-metric analysis.
Go to : 
REFERENCES
(1). Pieroni L. G., de Rezende F. M., Ximenes V. F., Dokkedal A. L.Molecules. 2011; 16:9439–9450.
(2). Susanti D., Sirat H. M., Ahmad F., Ali R. M., Aimi N., Kitajima M.Food Chem. 2007; 103:710–716.
(3). Nono R. N., Barboni L., Teponno R. B., Quassinti L., Bramucci M., Vitali L. A., Petrelli D., Lupidi G., Tapondjou A. L. Afr. J.Bot. 2014; 93:19–26.
(4). Cheng J. T., Hsu F. L., Chen H. F.Planta Med. 1993; 59:405–407.
(5). Amalraj T., Ignacimuthu S. J.Ethnopharmacol. 1998; 62:247–250.
(6). Ishii R., Saito K., Horie M., Shibano T., Kitanaka S., Amano F.Biol. Pharm. Bull. 1999; 22:647–653.
(7). Nicholl D. S., Daniels H. M., Ira Thabrew M., Grayer R. J., Simmonds M. S., Hughes R. D. J.Ethnopharmacol. 2001; 78:39–44.
(8). Ventura C. P., Braga de Oliveira A., Braga F. C.Rev. Bras. Farma. 2007; 17:17–22.
(9). Yoshida T., Arioka H., Fujita T., Chen X. M., Okuda T.Phytochemistry. 1994; 37:863–866.
(10). Isaza J. H., Ito H., Yoshida T.Phytochemistry. 2001; 58:321–327.
(11). Calderón A. I., Terreaux C., Schenk K., Pattison P., Burdette J. E., Pezzuto J. M., Gupta M. P., Hostettmann K. J.Nat. Prod. 2002; 65:1749–1753.
(12). Morimoto T., Nagatsu A., Murakami N., Sakakibara J., Tokuda H., Nishino H., Iwashima A.Phytochemistry. 1995; 40:1433–1437.
(13). Saeidnia S., Gohari A. R., Malmir M., Moradi-Afrapoli F., Ajani Y. J.Med. Plants. 2011; 1:41–47.
(14). Runyoro D. K. B., Srivastava S. K., Darokar M. P., Olipa N. D., Joseph C. C., Matee M. I.Med. Chem. Res. 2013; 22:1258–1262.
(15). Xu S., Shang M. Y., Liu G. X., Xu F., Wang X., Shou C. C., Cai S. Q.Molecule s. 2013; 18:5265–5287.
(16). Bisoli E., Garcez W. S., Hamerski L., Tieppo C., Garcez F. R.Molecules. 2008; 13:2717–2728.
(17). Xu H. X., Zeng F. Q., Wan M., Sim K. Y. J.Nat. Prod. 1996; 59:643–645.
(18). Maeda H., Matsuo Y., Tanaka T., Kouno I.Chem. Pharm. Bull. 2009; 57:421–423.
(19). Peng Z. F., Strack D., Baumert A., Subramaniam R., Goh N. K., Chia T. F., Tan S. N., Chia L. S.Phytochemistry. 2003; 62:219–228.
(20). Agrawal P. K.Phytochemistry. 1992; 31:3307–3330.
(21). Massiot G., Lavaud C.Studies in Natural Products Chemistry: Vol. 15; Elsevier Sciences, Amsterdam. 1995; 187–224.
(22). Iwashina T., Smirnov S. V., Damdinsuren O., Kondo K.Bull. Natl. Mus. Nat. Sci. 2012; 38:189–195.
Go to : 
Table 1.
1 H-NMR (CD3 OD, 500 MHz) and 13 C-NMR (CD3 OD,125 MHz) data of compound 1 (J in Hz)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download