Introduction
Endophytes are microorganisms which live in the internal plant tissues for all or part of their life span.1 They are considered to have complex associations with their host plants and other external environments.2 Due to their chemical or biological diversities, endophytic fungi have been recognized as new sources for discovery of structurally novel natural product leads.3 Halophytes are salt-resistant plants that can grow in waters of high salinity. It has been suggested that endophytes from halophytes contribute to the improvement of salt tolerance of the host plant.4 However, the studies about secondary metabolites produced by endophytes derived from halophytes are limited.
In the course of investigating chemical constituents of endophytic fungi, the fungal strain Gibberella moniliformis (JS1055) was isolated from a halophyte Vitex rotundifolia. G. moniliformis has been reported to produce various novel secondary metabolites such as a trioleoylglycerol (triolein), naphtoquinone (lawsone), and tricarballylic acids (Fumonisin A - C and P).456
Herein we performed a chemical investigation of EtOAc extract of an endophytic fungus G. moniliformis (JS1055) derived from V. rotundifolia and yielded the isolation of nine compounds (1 - 9): three isochromanone derivatives (1 - 3), two sterols (4 and 5), a fatty acid (6), a pyranonaphtoquinone (7), a pyridinecarboxylic acid (8), and a nucleoside (9).
Experimental
General experimental procedures
The high-resolution electrospray ionization mass spectra (HR-ESI-MS) were obtained using a Waters Xevo G2 QTOF mass spectrometer (Waters Co., Milford, MA, USA) and the LCQ Advantage trap mass spectrometer (Thermo Finnigan, San Jose, CA, USA) equipped with electrospray ionization (ESI) source. The NMR spectra, including 1H-1H COSY, HMQC, HMBC experiments, were recorded using Varian 500 spectrometer (1H, 500 MHz; 13C, 125 MHz) with chemical shifts given in ppm (δ). Column chromatography was performed using silica gel (Kieselgel 60, 70 - 230 and 230 - 400 mesh, Merck, Darmstadt, Germany) and Sephadex LH-20 (Pharmacia Co.) resins. Thin-layer chromatography (TLC) was performed using precoated silica gel 60 F254 and RP-18 F254S plates (both 0.25 mm, Merck, Darmstadt, Germany). The semi-preparative HPLC was performed using a Waters 600 controller (Waters, USA) with a 996 PDA detector using a ZORBAX SB-C18 column (21.2 mm × 25 cm, Agilent Technologies, USA).
Fungal materials
The fungal strain JS1055 were isolated from V. rotundifolia, collected from Suncheon, South Korea. The fungal strain JS1055 was identified as G. moniliformis by National Institute of Biological Resources (Incheon, South Korea). Parts of the material were deposited in College of Pharmacy, Duksung women's University. Plant tissues were divided into small pieces (0.5 × 0.5 cm) and their surfaces were stored in 2% sodium hypochlorite for 1 min and 70% ethanol for 1 min and then washed by sterilized distilled water. Fungal strains were grown out from the plant tissues for 7 days' incubation on malt extract agar (Difco) added to 50 ppm kanamycin, 50 ppm chloramphenicol, and 50 ppm Rose Bengal at 22 ℃. Fungal strains were cultured by transferring actively growing edges to a new potato dextrose agar (Difco) and were stored as 20% glycerol stocks in a liquid nitrogen tank.
Extraction and isolation
The fungal cultures were grown in Erlenmeyer flasks (20 × 500 mL) containing rice media. After autoclaving at 121 ℃ for 20 min and then cooling to room temperature, each flask was inoculated and then incubated at 28 ℃ under static conditions. After 21 days, the fermentation was stopped by adding 500 mL of EtOAc to each flask. The extraction was completed after the flasks had been sonicated for an hour three times. The EtOAc solution was evaporated under reduced pressure at 40 ℃ to give EtOAc extract (12.1 g). The EtOAc extract was subjected to vacuum liquid chromatography with the step gradient system (n-hexane- EtOAc 100:0 to 5:1; then CHCl3-MeOH 50:1 to 0:100 ) to give 8 fractions (Fr.1~8). Fraction 5 (410 mg) was separated by semi-preparative HPLC with ACN-H2O (75:25, 3 mL/min) solvent system to give seven fractions (Fr.5-1~7) using a Phenomax Luna 5u C18 column (250 × 10 mm, 5 µm). Compound 1 (2.0 mg) was isolated from fraction 5-5 by Sephadex LH-20 (MeOH). Compound 2 (0.7 mg) was purified from fraction 5-2 through Sephadex LH-20 (MeOH) and then semi-preparative HPLC with ACN-H2O (25:75 → 95:5, 3 mL/min). Compound 6 (2.1 mg) was obtained by recrystallization from the subfraction 5-7. Fraction 4 was separated by MPLC with n-hexaneacetone (100:0 to 5:1) solvent system to give four fractions (Fr.4-1~4-4). Compound 3 (0.2 mg) was obtained from Fr.4-2 and compounds 4 (0.5 mg) and 5 (0.3 mg) were isolated from Fr.4-3 by semi-preparative HPLC (ACN-H2O 70:30 → 100:0, 3 mL/min), respectively. Compound 7 (0.5 mg) was isolated from the fraction 7 through Sephadex LH-20 (MeOH) and then semi-preparative HPLC (ACN-H2O, 25:75 → 95:5, 3 mL/min). Fraction 8 was separated by MPLC with n-hexane-acetone (100:0 to 5:1) solvent system to give four fractions (Fr.8-1~8-7). Compound 8 (5.9 mg) was isolated by semi-preparative HPLC (ACN-H2O, 5:95 → 65:35, 3 mL/min) from Fr.8-4 and 9 (1.5 mg) was purified from Fr.8-6 by the same HPLC condition.
7-Butyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one (1)
White amorphous powder; HR-ESI-MS m/z: 305.1750 [M+H]+; CD (c 0.03, MeOH) Δε (nm): −3.16 (211), +1.72 (249), −3.45 (273), +0.17 (321); 1H-NMR (CD3OD, 500 MHz): δ 6.21 (1H, s, H-5), 5.50 (1H, m, H-12), 5.48 (1H, m, H-11), 4.49 (1H, m, H-3), 2.83 (2H, m, H-4), 2.58 (2H, t, J = 7.4 Hz, H-14), 2.17 (2H, m, H-10), 1.85 (1H, m, H-9a), 1.74 (1H, m, H-9b), 1.64 (3H, d, J = 6.0 Hz, CH3-13), 1.46 (2H, m, H-15), 1.35 (2H, m, H-16), 0.92 (3H, t, J = 7.3 Hz, CH3-17); 13C-NMR (CD3OD, 125 MHz): δ 170.8 (C-1), 162.3 (C-6), 161.8 (C-8), 138.6 (C-4a), 129.7 (C-11), 125.6 (C-12), 114.5 (C-7), 105.5 (C-5), 99.8 (C-8a), 78.8 (C-3), 34.3 (C-9), 32.3 (C-4), 30.6 (C-15), 27.5 (C-10), 22.4 (C-16), 21.7 (C-14), 16.7 (C-13), 13.0 (C-17).
7-Butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one (2)
White amorphous powder; ESI-MS m/z: 303.2 [M+H]+, 325.3 [M+Na]+; CD (c 0.03, MeOH) Δε (nm): −3.16 (211), +0.35 (249), −1.22 (273), +0.24 (307); 1H-NMR (CD3OD, 500 MHz): δ 6.22 (1H, s, H-5), 5.51 (1H, m, H-12), 5.49 (1H, m, H-15), 5.46 (1H, m, H-11), 5.43 (1H, m, H-16), 4.49 (1H, m, H-3), 3.24 (2H, d, J = 6.3 Hz, H-14), 2.83 (2H, m, H-4), 2.02 (2H, m, H-10), 1.85 (1H, m, H-9a), 1.74 (1H, m, H-9b), 1.64 (3H, d, J = 6.0 Hz, CH3-13), 1.58 (3H, d, J = 6.3 Hz, CH3-17); 13C-NMR (CD3OD, 125 MHz): δ 172.1 (C-1), 163.6 (C-6), 163.1 (C-8), 140.4 (C-4a), 131.1 (C-11), 129.6 (C-15), 127.0 (C-12), 125.8 (C-16), 114.2 (C-7), 107.0 (C-5), 101.3 (C-8a), 80.2 (C-3), 35.7 (C-9), 33.7 (C-4), 28.9 (C-10), 26.4 (C-14), 18.1 (C-13), 18.0 (C-17).
7-Butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one (3)
White amorphous powder; ESI-MS m/z: 307.2 [M+H]+; CD (c 0.03, MeOH) Δε (nm): −5.74 (207), +0.74 (249), −1.65 (274), +0.04 (299); 1H-NMR (CD3OD, 500 MHz): δ 6.21 (1H, s, H-5), 4.50 (1H, m, H-3), 2.83 (2H, m, H-4), 2.60 (2H, t, J = 7.5 Hz, H-14), 1.81 (2H, m, H-9), 1.73 (2H, m, H-10), 1.59 (2H, m, H-11), 1.46 (2H, m, H-15), 1.38 (2H, m, H-12), 1.37 (2H, m, H-14), 1.36 (2H, m, H-16), 0.94 (3H, t, J = 7.0 Hz, CH3-13), 0.93 (3H, d, J = 7.3 Hz, CH3-17).
5α,8α-Epidioxyergosta-6,9(11),22-trien-3-ol (=9,11-dehydroergosterol peroxide) (4)
White amorphous powder; ESI-MS m/z: 449.6 [M+Na]+; 1H-NMR (CD3OD, 500 MHz): δ 6.66 (1H, d, J = 8.3 Hz, H-7), 6.32 (1H, d, J = 8.3 Hz, H-6), 5.49 (1H, dd, J = 5.6, 2.0 Hz, H-11), 5.27 (1H, m, H-22), 5.20 (1H, m, H-23), 3.82 (1H, m, H-3), 2.31-1.20 (22H, m, H-1, 2, 4, 12, 14, 15, 16, 17, 20, 24, 25), 1.12 (3H, s, CH3-19), 1.03 (3H, d, J = 6.5 Hz, CH3-21), 0.95 (3H, s, CH3-28), 0.88 (3H, d, J = 6.7 Hz, CH3-26), 0.85 (3H, d, J = 6.7 Hz, CH3-27), 0.78 (3H, s, CH3-18); 13C-NMR (CD3OD, 125 MHz): δ 142.8 (C-9), 135.4 (C-6), 135.3 (C-22), 132.1 (C-23), 130.3 (C-7), 119.3 (C-11), 82.6 (C-5), 78.3 (C-8), 65.4 (C-3), 55.8 (C-17), 48.2 (C-14), 43.4 (C-13), 42.9 (C-24), 41.0 (C-12), 39.9 (C-20), 37.8 (C-10), 35.5 (C-4), 32.9 (C-25), 32.3 (C-1), 29.9 (C-2), 28.4 (C-16), 24.5 (C-19), 20.4 (C-15), 19.8 (C-21), 19.0 (C-26), 18.7 (C-27), 16.8 (C-28), 11.9 (C-18).
Ergosterol peroxide (5)
White amorphous powder; ESI-MS m/z: 451.8 [M+Na]+; 1H-NMR (CD3OD, 500 MHz): δ 6.52 (1H, d, J = 8.6 Hz, H-7), 6.25 (1H, d, J = 8.6 Hz, H-6), 5.24 (1H, dd, J = 15.3, 8.3 Hz, H-23), 5.17 (1H, dd, J = 15.0, 7.9 Hz, H-22), 3.76 (1H, m, H-3), 2.04 (1H, m, H-20), 1.97 (1H, m, H-12a), 1.96 (1H, m, H-4a), 1.90 (1H, m, H-4b), 1.87 (1H, m, H-1a), 1.83 (1H, m, H-24), 1.77 (1H, m, H-2a), 1.74 (1H, m, H-16a), 1.69 (1H, m, H-1b), 1.54 (1H, m, H-11a), 1.52 (1H, m, H-14), 1.51 (1H, m, H-2b), 1.51 (1H, m, H-15a), 1.46 (1H, m, H-25), 1.44 (1H, m, H-15b), 1.43 (1H, m, H-9), 1.38 (1H, m, H-16b), 1.25 (1H, m, H-11b), 1.24 (1H, m, H-12b), 1.24 (1H, m, H-17), 1.01 (3H, d, J = 6.7 Hz, CH3-21), 0.93 (3H, d, J = 7.0 Hz, CH3-28), 0.90 (3H, s, CH3-19), 0.85 (3H, d, J = 6.7 Hz, CH3-26), 0.84 (3H, s, CH3-18), 0.83 (3H, d, J = 6.9 Hz, CH3-27).
Tetradecanoic acid (6)
White amorphous powder; ESI-MS m/z: 251.3 [M+Na]+; 1H-NMR (CD3OD, 500 MHz): δ 2.26 (2H, t, J = 7.5 Hz, H-2), 1.58 (2H, m, H-3), 1.33 - 1.26 (16H, m, H-4, 5, 6, 7, 8, 9, 10, and 11), 1.29 (2H, m, H-13), 1.27 (2H, m, H-12), 0.89 (3H, t, J = 7.2 Hz, CH3-14); 13C-NMR (CD3OD, 125 MHz): δ 176.5 (C-1), 33.7 (C-2), 31.7 (C-12), 29.39-28.86 (C-4, 5, 6, 7, 8, 9, 10, and 11), 24.8 (C-3), 22.3 (C-13), 13.0 (C-14).
8-O-Methylfusarubin (7)
Red amorphous powder; HR-ESI-MS m/z: 321.0974 [M+H]+; ESI-MS m/z: 321.0 [M+H]+, 343.0 [M+Na]+; 1H-NMR (CD3OD, 500 MHz): δ 6.99 (1H, s, H-7), 4.62 (2H, d, J = 4.8 Hz, H-1), 4.02 (3H, s, OCH3), 3.98 (3H, s, OCH3), 2.76 (1H, m), 2.50 (1H, m), 1.52 (3H, s, H-15); 13C-NMR (CD3OD, 125 MHz): δ 182.4 (C-9 and C-10), 158.5 (C-8), 158.2 (C-6), 150.1 (C-5), 146.7 (C-13), 139.5 (C-14), 115.6 (C-11), 110.8 (C-12), 104.6 (C-7), 95.4 (C-3), 59.8 (C-1), 57.6 (OCH3-16 and 17), 33.2 (C-4), 29.5 (C-15).
Nicotinic acid (8)
Colorless amorphous powder; ESIMS m/z: 124.1 [M+H]+; 1H-NMR (CD3OD, 500 MHz): δ 9.01 (1H, d, J = 1.5 Hz, H-2), 8.69 (1H, dd, J = 5.0, 1.5 Hz, H-4), 8.30 (1H, dt, J = 8.0, 2.0 Hz, H-6), 7.54 (1H, ddd, J = 18.0, 5.0, 0.8 Hz, H-5); 13C-NMR (CD3OD, 125 MHz): δ 170.3 (C-7), 153.3 (C-4), 149.9 (C-2), 137.8 (C-6), 131.9 (C-1), 125.6 (C-5).
Result and Discussion
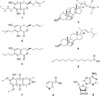
The chemical investigation of the EtOAc extract of an endophytic fungus G. moniliformis JS1055 resulted in the isolation of nine compounds (1 - 9), including 7-butyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one (1), 7-butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one (2), 7-butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one (3), 5α,8α-epidioxyergosta-6,9(11),22-trien-3-ol (=9,11-dehydroergosterol peroxide) (4), ergosterol peroxide (5), tetradecanoic acid (6), 8-O-methylfusarubin (7), nicotinic acid (8), and adenosine (9) (Fig. 1). The structures of isolated compounds were identified based on spectroscopic data including 1D, 2D NMR, and ESIMS, by comparing their data values with those of previously reported literatures.78910111213 All these isolated compounds are firstly reported from this endophytic fungus.
Compound 1 was obtained as a white amorphous powder and its molecular formula was determined to be C18H24O4 from the ion peak [M+H]+ at m/z 305.1750 (calcd for C18H24O4, 305.1753) in the positive mode HR-ESI-MS. The 1H NMR spectrum of 1 displayed signals for an aromatic proton [δH 6.21 (1H, s)], two olefinic protons [δH 5.50 (1H, m) and 5.48 (1H, m)], an oxygenated methine proton [δH 4.49 (1H, m)], six methylenes and two methyl protons [δH 1.64 (3H, d, J = 6.0 Hz) and 0.92 (3H, t, J = 7.3 Hz)]. The 13C NMR spectrum exhibited 18 carbon signals for two olefinic carbons at δC 129.7 and 125.6, an aromatic methine at δC 105.5, an oxygenated methine at δC 78.8, six methylenes at δC 34.3, 32.3, 30.6, 27.5, 22.4, and 21.7, two methyls at δC 16.7 and 13.0, and six quaternary carbons. From these 1D NMR data, 1 was suggested to be an isochroman-1-one derivative which had a pentasubsituted aromatic ring in the structure. The 1H-1H COSY correlations indicated the partial fragments of structure, C(13)H3-C(12)H-C(11)H-C(10)H2-C(9)H2-C(3)H-C(4)H2 and C(14)H2-C(15)H2-C(16)H2-C(17)H3 (Fig. 2). The HMBC correlations of H-3 at δH 4.49 with C-9, C-10, and C-4a and of H2-14 at δH 2.58 with C-6, C-7, and C-8 allowed for the connection of the above partial fragments with the isochroman-1-one backbone. Furthermore, a stereochemistry at C-3 was determined by the measurement of CD spectrum. According to a literature, an isochromanone structure shows the Cotton effect attributed to a benzoic ester chromophore.14 From the negative Cotton effect at 273 nm, the absolute configuration of C-3 was assigned to be R.11 From these spectral data, 1 was elucidated as 7-butyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one and its NMR data were in good agreement with the literature values.11
The 1H-NMR spectra of 2 and 3 were similar to those of 1 except for the regions of olefinic groups. The 1HNMR spectrum of 2 exhibited an additional presence of two olefinic protons at δH 5.5 whereas that of 3 showed an absence of olefinic protons. Moreover, the CD spectra of 2 and 3 showed negative Cotton effect at 273 nm and 274 nm, respectively, indicating that C-3 of these compounds had both R configurations. By careful comparison of those data with previously reported literature, compounds 2 and 3 were assigned as 7-but-15-enyl-6,8-dihydroxy-3(R)-pent-11-enylisochroman-1-one and 7-butyl-6,8-dihydroxy-3(R)-pentylisochroman-1-one, respectively.11
Compound 7 was obtained as a yellowish amorphous powder and its molecular formula was established as C16H16O7 by the positive ion mode HR-ESI-MS data at m/z 321.0974 (calcd for C16H17O7, 321.0974). The 1H NMR spectrum of 7 showed an aromatic proton [δH 6.99 (1H, s)], an oxymethylene proton [δH 4.62 (2H, d, J = 4.8 Hz)], two methoxy protons [δH 4.02 (3H, s) and 3.98 (3H, s)], a methylene [δH 2.76 (1H, m) and 2.50 (1H, m)], and a methyl proton [δH 1.52 (3H, s)]. The 13C NMR spectrum exhibited 16 carbon resonances including an aromatic methine (δC 104.6), an oxymethylene (δC 59.8), two methoxy (δC 57.6, overlapped), a methylene (δC 33.2), and nine quaternary carbons. The HMBC correlations of the aromatic methine proton at δH 6.99 with carbons at δC 110.8, 150.1, 158.2, and 182.4 indicated the presence of 1,4-naphthoquinone with substitutions of two methoxy groups and a hydroxyl group in the aromatic ring. In addition, the HMBC correlations of the methylene protons (δH 4.62) with C-3 (δC 95.4), C-14 (δC 139.5), and C-13 (δC 146.7) and of the methyl (δH 1.52) with C-4 (δC 33.2), C-3 (δC 95.4), and C-14 (δC 139.5) revealed a 3-hydroxy-3-methyl pyran moiety was attached to a 1,4-naphthoquinone skeleton. Therefore, the structure of 7 was deduced as 8-O-methylfusarubin.10
In summary, an endophytic fungus G. moniliformis (JS1055) was isolated from a tissue of a halophyte V. rotundifolia and the cultures of the fungus was subjected to chemical investigation to consequently get nine metabolites (1 - 9). These findings would be helpful for understanding the chemical characteristics of G. moniliformis endophytic fungus and its future application in agriculture, medicine and food industry.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


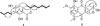
 XML Download
XML Download