Abstract
Chronic oxidative stress due to the accumulation of reactive oxygen species (ROS) in neuronal cells ultimately leads to neurodegenerative diseases. The use of natural therapies for the prevention of ROS-induced cell damage and for the treatment of neurodegenerative disorders has shown promising results. In this study, we evaluated the neuroprotective effects of the ethyl acetate (EtOAc) fraction of A. Okamotoanum against the hydrogen peroxide (H2O2)-induced oxidative stress in C6 glial cells. Results show that cell viability was decreased in cells incubated with H2O2, whereas the addition of EtOAc fraction treatments in such cells significantly increased viability. The EtOAc fraction showed the highest inhibitory activity against ROS production and it also decreased the expressions of inflammatory proteins including cyclooxygenase-2, inducible nitric oxide synthase and interleukin-1β. Furthermore, the EtOAc fraction inhibited apoptosis by regulating the protein expressions cleaved caspase -9, -3, poly ADP ribose polymerase, Bax and Bcl-2. Therefore, these results show that the EtOAc fraction of A. Okamotoanum exhibits neuroprotective effects against H2O2 induced oxidative damage by regulating the inflammatory reaction and apoptotic pathway.
Reactive oxygen species (ROS) and free radicals such as hydroxyl radical (·OH), nitric oxide radical (NO·), superoxide radical (O2-), and hydrogen peroxide (H2O2), are highly reactive byproducts of oxygen metabolism in cells.1 In the normal conditions, ROS are removed by antioxidant defense systems.2 However, during pathological conditions, there is an imbalance between the generation of free radical and antioxidant systems in cells resulting to oxidative stress. Ultimately, prolonged oxidative stress leads to many diseases including cancer, arthritis, autoimmune, and neurodegenerative disorders.3 In addition, the excessive accumulation of ROS in glial cells results to cell injury through the transcription of pro-inflammatory gene transcription and release of cytokines-interleukin (IL)-1β, and IL-6, thereby causing neuro-inflammation. Moreover, the released inflammatory cytokines leads to oxidative stress and mitochondrial dysfunction, which is main cause of neuronal cell death.4 Particularly the accumulation of H2O2 in neuronal cells results to the pathological process of acute and chronic neurotoxicity and neuronal apoptosis as it directly oxidizes lipids, proteins, and deoxyribonucleic acid.4
Acer okamotoanum (A. okamotoanum) is a perennial plant indigenous to Korea and is widely distributed in the mountains of Ulleung Island. Traditionally, its branches, leaves, and roots have been used as treatment for arthralgia and fractures. Phytochemical analysis of A. okamotoanum revealed that it contains many bioactive compounds such as quercetin, kaempferol, tannin, gallic acid, cleomiscin A and C.56 Recent studies have shown that A. okamotoanum exhibited numerous biological activities which include immunomodulatory effects, skin whitening, anti-HIV-1 integrase, anti-cancer, antioxidant, and anti-atherosclerosis activity.678910 Moreover, we previously reported that A. okamotoanum exhibited neuroprotective effects against amyloid beta (Aβ)-induced neurotoxicity. Particularly, we discovered that the ethyl acetate (EtOAc) fraction of A. okamotoanum showed the strongest inhibition against ROS in Aβ-induced C6 glial cells. The EtOAc fraction also exhibited strong in vitro radical scavenging activity and contains high total phenol and flavonoid content. However, the neuroprotective effect of A. okamotoanum against oxidative damage in glial cells is yet to be demonstrated. Therefore, this study aimed to investigate the antioxidative and neuroprotective effects of the EtOAc fraction of A. okamotoanum against H2O2-induced oxidative stress in C6 glial cells.
A. okamotoanum was provided by Korea National Arboretum. The samples were collected from Ulleung Island, Korea. A voucher specimen (No. LEE 2014-04) was deposited at the herbarium of the Department of Integrative Plant Science, Chung-Ang University.
Sodium nitrite (NaNO2), sodium hydroxide (NaOH), aluminium chloride (AlCl3) and H2O2 were purchased from Sigma Chemicals Co. (St. Louis, USA) or Merck (Darmstall, Germany) and Junsei Chemical Co. (Tokyo, Japan). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) and 2′,7′-dichlorofluorescin diacetate (DCF-DA) were also purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were obtained from Welgene (Daegu, Korea). The lysis protein extraction buffer and enhanced chemiluminescence (ECL) reagents were purchased from Elpis Biotech (Daejeon, Korea). Phosphatase inhibitor cocktail was purchased from Calbiochem-Novabiochem (La Jolla, CA, USA). As shown in Table 1, protein assay kit, primary and secondary antibodies were purchased from Sigma Chemical Co. (St Louis, MO, USA) or Cell Signaling Technology (Beverly, MA), and Santa Cruz Biotechnology (Santa Cruz, MA, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from Millipore Co. (Billerica, MA, USA).
Dried A. okamotoanum (995.4 g) was extracted with methanol (MeOH) at 65 – 75℃ for 3 h. The resulting extract solution was evaporated to dryness in vacuo to obtain the MeOH extract (176.1 g). Afterwards, the dried MeOH extract of A. okamotoanum was suspended in distilled water and partitioned with EtOAc. The MeOH extract and EtOAc fraction (35 g) were stored at −80 ℃ before use.
A C6 glial cell line was obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were retained in a culture flask (T75) supplemented with DMEM containing 10% FBS and were incubated in a humidified atmosphere (5% CO2, 95% air) at 37 ℃. Cells were sub-cultured with 0.05% trypsin-EDTA in calcium and magnesium-free phosphate buffer upon reaching confluence. Each experiment was repeated three times (n = 3).
C6 glial cells were seeded in a 96-well microtiter plate at a cell density of 5 × 104 cells/mL. The cells were treated with different concentrations of the test extracts and incubated for 2 h (5% CO2, 37 ℃). After 2 h, 300 µM H2O2 was added to the test wells and the cells were incubated for another 24 h. Cell viability was then assessed using the MTT colorimetric assay.12 In detail, each test well was added with 200 µL of MTT solution (5 mg/mL) and the cells were incubated again for 4 h. After which, the culture medium was withdrawn and 200 µL DMSO was added to every test well to solubilize the incorporated formazan crystals that had formed in the MTT-incubated glial cells. Absorbance was measured at 540 nm using a microplate reader.
C6 glial cells were seeded at a density of 5 × 104 cells/mL in a 96-well black culture microplate. The cells were treated with different concentrations of the test extracts and incubated for 2 h (5% CO2, 37 ℃). After 2 h, 300 µM H2O2 was added to the test wells and the cells were incubated for another 24 h. After which, the intracellular production of ROS in C6 glial cells was determined by measuring the oxidation of DCF-DA to DCF.13 In detail, 20 µM DCF-DA was added to each test well and the cells were incubated again for 30 min. Fluorescence was then measured using a fluorescence spectrophotometer at excitation and emission wavelengths of 400 nm and 505 nm, respectively.
C6 glial cells were seeded at a density of 1 × 105 cells/mL in 100 mm2 cell culture dishes. The cells were treated with different concentrations of the test extracts and incubated for 2 h (5% CO2, 37 ℃). After 2 h, 300 µM H2O2 was added to the test wells and the cells were incubated for another 24 h. After which, the cells were harvested by adding phosphate buffered saline (PBS) and scraping the cells from the culture dish. The cells suspended in PBS were centrifuged at 2,000 rpm for 5min at 4 ℃. The cell pellet was then resuspended in 50 µL ice-cold lysis buffer including protease and phosphatase inhibitor cocktails and was incubated on ice for 1 h. Centrifugation of the cell solution at 12,000 rpm for 30 min followed, and the supernatant was then collected and stored at −80 ℃. The protein concentration was measured using a protein assay. Proteins were separated on a 10 or 13% sodium dodecyl sulfate-polyacrylamide gel, and then transferred to PVDF membrane that was blocked with 5% skim milk in PBS-T for 1 h at 25 ℃. The membrane was exposed to primary antibodies (Table 1) overnight at 4 ℃. Subsequently, the membrane was washed with PBS-T and incubated with appropriate secondary antibodies (Table 1) in PBS-T for 1 h at 25 ℃. Bands were developed using the ECL detection method according to the manufacturer's specification and were visualized on a photographic film for several minutes. Bands were scanned using Davinch-Chemi™ (Davinch-K, Seoul, Korea). Beta-actin was used as a loading control.
SAS software (version 6.0, SAS Institute, Cary, NC, USA) was used to perform statistical analyses. The data are represented as the mean ± standard deviation (SD). ANOVA and Duncan's multiple range test were performed to determine significant differences among test groups. P values <0.05 were considered as significantly different.
Fig. 1, shows the effects of the EtOAc fraction treatments on the viability of C6 glial cells incubated with H2O2. Cell viability in the normal group was 100%, whereas a significant decline to 49.1% cell viability was observed in cells incubated with H2O2 alone. On the other hand, the addition of the EtOAc fraction in C6 glial cells incubated with H2O2 improved cell viability at all treatment concentrations. The EtOAc fraction treatments at low doses (5 and 25 µg/mL) showed similar values with the normal group.
The inhibitory effects of the EtOAc fraction of A. okamotoanum on ROS production in C6 glial cells incubated with H2O2 was determined using the DCF-DA assay. The results of the experiment are shown in Fig. 2. An increase in ROS production was observed in the control group compared to the normal group. This indicates that oxidative stress is generated in cells incubated with H2O2 as seen from the increased production of ROS. When the cells were treated with 5, 25, 50 and 100 µg/mL of EtOAc fraction, the ROS production declined to 84.01%, 81.89%, 80.47% and 77.28%, respectively.
The effects of the EtOAc fraction treatments on the expression of inflammation-related proteins in C6 glial cells incubated with H2O2 was examined (Fig. 3). The cells were treated with 2.5, 5 and 25 µg/mL of EtOAc fraction and 300 µM of H2O2. Hydrogen peroxide-treated control group decreased the expression of IκB-α protein, whereas cells treated with EtOAc fraction exhibited an up-regulatory effect against the down-expression of IκB-α protein. In addition, the control group showed higher protein expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (Cox-2) and IL-1β than the normal group, whereas the EtOAc fraction-treated groups (2.5, 5 and 25 µg/mL) showed a dose-dependent decline in iNOS, Cox-2 and IL-1β expression.
We examined whether EtOAc fraction affects the protein expressions of pro-apoptotic and anti-apoptotic proteins in H2O2-treated C6 glial cells. The protein expression levels of Bax protein (pro-apoptotic) and Bcl-2 protein (anti-apoptotic) was determined using western blot assay (Fig. 4.A). The Bax/Bcl-2 ratio was approximately up-regulated by 2-folds in H2O2-treated control group, compared with the H2O2-nontreated normal group, whereas the EtOAc fraction-treated groups exhibited a significantly lower Bax/Bcl-2 ratio. These results indicated that the EtOAc fraction from A. okamotoanum has the potential to inhibit H2O2-induced cell death in C6 glial cells.
To investigate the further protective mechanism of of A. okamotoanum against apoptosis, we examined the effects of the different treatments of the EtOAc fraction on the protein expression levels of cleaved caspase 9, cleaved caspase 3 and cleaved poly ADP ribose polymerase (PARP) in H2O2 treated C6 glial cells (Fig. 5). The H2O2-treated control group significantly upregulated the expression of cleaved caspase 9, cleaved caspase 3, and cleaved PARP compared with H2O2-nontreated normal group. However, the increased expressions of cleaved caspase 9, cleaved caspase 3, and cleaved PARP were significantly and dose-dependently inhibited by the treatments of EtOAc fraction from A. okamatoanum. Particularly, cleaved caspase 9 and cleaved PARP expression were down regulated almost to the normal levels at the concentration of 25 µg/mL. These results suggest that A. okamotoanum was associated with the inactivation of the apoptosis pathways in H2O2-induced oxidative stress in C6 glial cell by inhibiting the expression of apoptotic proteins including cleaved caspase 9, cleaved caspase 3, and cleaved PARP.
Neurons are sensitive to urgent oxidative stress.14 ROS are related to apoptotic cell death caused by intracellular micro-environmental changes, which is one of the critical causes of degenerative diseases, including dementia.15 In particular, H2O2 has been widely used to induce oxidative stress in vitro.16 The accumulation of H2O2 causes apoptosis in neurons by damaging proteins and lipids, and causing mitochondrial membrane dysfunction and DNA damage.1416
Therefore, inhibiting ROS formation can be an effective method to protect neuronal cell damage that leads to degenerative diseases and aging-related cognitive impairment.1517 In addition, glial cells supply structural support to promote the metabolisms and protect neuronal cells by generating neurotrophic factors that are potentially helpful to the survival of immature neurons.18 Thus, glial cell activation may play critical functions in the commencement and progression of various neurodegenerative diseases.
A. okamotoanum has long been used in traditional medicine to treat various diseases and recent studies have also shown that it exhibits several biological activities. These include its use an anti-cancer, anti-HIV-1 integrase, antioxidant, anti-herpetic activities and in skin whitening.78910 However there are no existing literature concerning the protective effects of A. okamotoanum against oxidative stress in C6 glial cells. In this study, we examined the molecular mechanisms underlying the neuroprotective effects of A. okamotoanum against H2O2-induced oxidative stress in C6 glial cells by determining its effects on the inflammatory and apoptotic pathways.
We examined the protective effects of the EtOAc fraction of A. okamotoanumon H2O2-induced oxidative stress and neuronal cell damage using C6 glial cells as in vitro model systems. From our previous study, the EtOAc fraction was confirmed as the active fraction of A. okamotoanum which have also shown strong antioxidative effects.11 Prior to performing the MTT cell viability assay, a cytotoxicity test on the EtOAc fraction of A. okamotoanumon C6 glial cells was carried out. EtOAc treatments at a dosage range of 5 - 100 µg/mL and a 24-hr incubation period showed no significant cytotoxicity in C6 glial cells (data not shown). In this study, we have shown that all the groups treated with the EtOAc fraction significantly increased cell viability compared to H2O2-treated control group. However, the protective effects of the EtOAc fraction did not increase proportionally to treatment concentrations.
To determine whether EtOAc fraction of A. okamotoanum inhibited neuronal apoptosis by scavenging ROS, the DCF-DA assay was used to measure changes in ROS production. An increased ROS fluorescence in H2O2-treated C6 glial cells was observed indicating an increase of intracellular ROS, whereas cells treated with EtOAc inhibited ROS production. Mahesh and Kim suggested that H2O2 produced in the pathological process of acute and chronic neurotoxicity and increased the levels of ROS in C6 glial cells.4 Our results revealed that the EtOAc fraction of A. okamotoanum significantly inhibited ROS formation and prevented H2O2-induced cell damage in neuronal cells due to its antioxidant activity. This shows that the neuroprotective role of the EtOAc fraction can be attributed to its antioxidant activity.
Nuclear factor-κB (NF-κB) is responsible for the regulation of several target genes related to the inflammatory response, immune reactions, and apoptosis. NF-κB binds with dimers of p50 and p65 subunits that exist as inactive types in most cells.19 Different dimeric structures are bound to a protein inhibitory subunit of IκB that keeps them inactive in the cytoplasm. The translocation to the nucleus is feasible only when IκB dissociates from the dimer. In addition, IκB probably blocks not only this translocation but also NF-κB DNA-binding activity.1920 Upon the exposure of cultured cells to oxidative stress, phosphorylated IκB-α is degraded through selective ubiquitination, and NF-κB is released and translocated to the nucleus.16 Other transcription factors bind to specific DNA fragments and then activates the up-regulation of their target molecules such as cytokines and apoptosis-related proteins. Protein expressions of iNOS and Cox-2 have significant roles in the process of tissues damage or inflammation.2122 Although activation of iNOS and Cox-2 may donate a precise profit to the organism, overexpression of either iNOS or Cox-2 has been involved in the outbreak of various disorders, such as diverse as septic shock, acute and chronic neurodegenerative disease and rheumatoid arthritis.2223 The treatment of H2O2showed higher levels of crucial inflammatory indicator such as NO, through up-regulation of Cox-2 and iNOS in C6 cells.2425 Therefore, the demolition of normal mechanisms of NF-κB action may result in numerous abnormalities and pathologies in the organism, including inflammatory diseases, atherosclerosis, toxic/septic shock, cancers, and neurodegenerative diseases.2627 In this study, we investigated whether EtOAc fraction from A. okamotoanum protects against inflammatory target proteins of IκB-α, Cox-2, iNOS and IL-1β induced by H2O2. The results demonstrated that EtOAc fraction from A. okamotoanum regulated the expressions of IκB-α, Cox-2, iNOS and IL-1β caused by H2O2 treatment in C6 glial cells. In addition, previous researches reported anti-inflammatory effects of A. okamotoanum by down-regulations of prostaglandin (PG) E2 and NO concentration in the cellular and in vivo mice model.828 Therefore, we demonstrated protective effects of EtOAc fraction from A. okamotoanum on H2O2-induced neuronal cell damage by regulation of inflammatory genes.
H2O2-induced apoptosis is linked to the activation of Bcl-2 and inactivation of Bax which are anti-apoptotic and pro-apoptotic proteins, respectively. Cytochrome c initiates a cascade of caspase activation that leads to apoptotic cell death.29 The up-regulation of intracellular Bax/Bcl-2 ratio occurs during apoptotic cell death. The ratio of pro-apoptotic proteins/anti-apoptotic proteins decides the sensitivity or resistance of cells to the process of apoptosis.30 In the present study, H2O2-treated control group significantly up-regulated the Bax/Bcl-2 ratio. On the other hand, the EtOAc fraction decreased the Bax/Bcl-2 ratio in a dose-dependent manner, indicating that it exhibited neuroprotective effects against H2O2-induced cell damage in glial cells by inhibiting apoptotic cell death.
Crucial target in ROS-induced cytotoxicity leads to changes in mitochondrial membrane permeability.31 It leads to mitochondrial dysfunctions and efflux of cytochrome c may lead to process of apoptotic cell death by reduction of mitochondria membrane potential in cells. Previous researches demonstrated a critical factor for the activation of caspase 3 by the activation of caspase 9, leading to cleavage of PARP from its full-length phase.32 Furthermore, cleaved PARP occurs in systems where ROS-induced DNA damage is so extensive that energy required for renovation is diverted elsewhere through apoptosis.2632 In this study, we measured cleaved caspase-9, cleaved caspase-3 and cleaved PARP levels in C6 glial cells to examine the protective effects of the EtOAc fraction on mitochondrial function in H2O2-induced apoptosis. We confirmed that the EtOAc fraction of A. okamotoanum down-regulated the expression of cleaved caspase-9, cleaved caspase-3 and cleaved PARP. These results indicate that the EtOAc fraction of A. okamotoanum inhibited H2O2-induced apoptosis.
In conclusion, the EtOAc fraction A. okamotoanum protected C6 glial cells from H2O2-induced oxidative stress by inhibiting apoptosis and decreasing ROS production. In addition, the protective effects of the EtOAc fraction against oxidative stress relates to the regulation of the expression of proteins associated with inflammation and apoptosis. The present research suggests that the EtOAc fraction of A. okamotoanum may be used as a potential neuroprotective agent for the treatment of neurodegenerative diseases.
Figures and Tables
 | Fig. 1The effects of the EtOAc fraction of A. okamotoanum on the cell viability of H2O2-treated C6 glial cells. Cells were pretreated with different concentrations (5, 25, 50, 100 µg/mL) of the EtOAc fraction of A. okamotoanum for 2 hr, and then incubated with 300 µM H2O2 for 24 hr. Values are shown as mean ± SD. a~e Means; different letters are significantly different (P<0.05) using the Duncan's multiple range test. |
 | Fig. 2The effects of the EtOAc fraction of A. okamotoanum on the ROS levels of H2O2-treated C6 glial cells. Cells were pretreated with different concentrations (5, 25, 50, 100 µg/mL) of the EtOAc fraction of A. okamotoanum for 2 hr, and then incubated with 300 µM H2O2 for 24 hr. Values are shown as mean ± SD. a~c Means; different letters are significantly different (P<0.05) using the Duncan's multiple range test. |
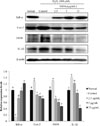 | Fig. 3The effects of the EtOAc fraction of A. okamotoanum on the expression of IκB-α, Cox-2, iNOS and IL-1β in H2O2-treated C6 glial cells. Cells were pretreated with different concentrations (2.5, 5, 25 µg/mL) of the EtOAc fraction of A. okamotoanum for 2 hr, and then incubated with 300 µM H2O2 for 24 hr. β-actin was used as loading control. Values are shown as the mean ± SD. a~e Means; different letters are significantly different (P<0.05) using the Duncan's multiple range test. |
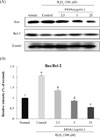 | Fig. 4The effects of the EtOAc fraction of A. okamotoanum on Bax and Bcl-2 expression (A) and the ratio of Bax/Bcl-2 (B) in H2O2-treated C6 glial cells. Cells were pretreated with different concentrations (2.5, 5, 25 µg/mL) of the EtOAc fraction of A. okamotoanum for 2 hr, and then incubated with 300 µM H2O2 for 24 hr. β-actin was used as loading control. Values are shown as the mean ± SD. a~e Means; different letters are significantly different (P<0.05) using the Duncan's multiple range test. |
 | Fig. 5The effects of the EtOAc fraction of A. okamotoanumon H2O2-induced increases cleaved caspase 9 (A), cleaved caspase 3 (B), and cleaved PARP (C) levels in C6 glial cells. Cells were pretreated with different concentrations (2.5, 5, 25 µg/mL) of the EtOAc fraction of A. okamotoanum for 2 hr, and then incubated with 300 µM H2O2 for 24 hr. β-actin was used as loading control. Values are shown as the mean ± SD. a~eMeans; different letters are significantly different (P<0.05) using the Duncan's multiple range test. |
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A1A01058868).
References
1. Heo SJ, Jeon YJ. J Appl Phycol. 2008; 20:1087–1095.
2. Ko SC, Kim D, Jeon Y. J Food Chem Toxicol. 2012; 50:2294–2302.
3. Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. Biomed Pharmacother. 2015; 74:101–110.
4. Qin B, Panickar KS, Anderson RA. Life Sci. 2014; 102:72–79.
5. Kim HJ, Woo ER, Shin CG, Park HJ. Nat Prod. 1998; 61:145–148.
6. Jin W, Thuong PT, Su ND, Min BS, Son KH, Chang HW, Kim HP, Kang SS, Sok DE, Bae K. Arch Pharm Res. 2007; 30:275–281.
7. Jeong MH, Ha JH, Oh SH, Kim SS, Lee HJ, Kang HY, Lee HY. Korean J Med Crop Sci. 2009; 17:407–411.
8. Jeong MH, Kim SS, Kim JS, Lee HJ, Choi GP, Lee HY. J Korean For Soc. 2010; 99:470–478.
9. Jin L, Han JG, Ha JH, Jeong HS, Kwon MC, Jeong MH, Lee HJ, Kang HY, Choi DH, Lee HY. Korean J Med Crop Sci. 2008; 16:427–433.
10. Qadir SA, Kim CH, Kwon MC, Lee HJ, Kang HY, Choi DH, Lee HY. Korean J Med Crop Sci. 2007; 15:405–410.
11. Choi SY, Kim JH, Lee JM, Lee SH, Cho EJ. J Appl Biol Chem. 2017; 60:141–147.
12. Mosmann T. J Immunol Methods. 1983; 65:55–63.
13. Ma WW, Hou CC, Zhou X, Yu HL, Xi YD, Ding J, Zhao X, Xiao R. Neurochem Res. 2013; 38:1315–1323.
14. Park JH, Kim CK, Lee SB, Lee KH, Cho SW, Ahn JY. Sci Rep. 2016; 6:21857.
15. Park HR, Lee H, Park H, Jeon JW, Cho WK, Ma JY. BMC Complement Altern Med. 2015; 15:171–181.
16. Kwon SH, Hong SI, Ma SX, Lee SY, Jang CG. Food Chem Toxicol. 2015; 80:41–51.
17. Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Psychopharmacology. 2015; 232:931–942.
18. Barnham KJ, Masters CL, Bush AI. Nat Rev Drug Discov. 2004; 3:205–214.
19. Malek R, Borowicz KK, Jargiello M, Czuczwar SJ. Pharmacol Rep. 2007; 59:25–33.
20. Janssen-Heininger YM, Poynter ME, Baeuerle PA. Free Radic Biol Med. 2000; 28:1317–1327.
21. Moneada S, Palmer RM, Higgs EA. Pharmacol Rev. 1991; 43:109–142.
22. Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB. Cancer Res. 1998; 58:717–723.
23. DuBois RN, Radhika A, Reddy BS, Entingh AJ. Gastroenterology. 1996; 110:1259–1262.
24. Ogura M, Takarada T, Nakamichi N, Kawagoe H, Sako A, Nakazato R, Yoneda Y. Neurochem Int. 2011; 58:504–511.
25. Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Gonçalves CA, Gottfried C. PLoS One. 2013; 8:e64372.
26. Pahl HL. Oncogene. 1999; 18:6853–6866.
27. Shi X, Dong Z, Huang C, Ma W, Liu K, Ye J, Chen F, Leonard SS, Ding M, Castranova V, Vallyathan V. Mol Cell Biochem. 1999; 194:63–70.

28. Choi SY, Lee J, Lee DG, Lee S, Cho EJ. Appl Biol Chem. 2017; 60:1–9.
29. Alamdary SZ, Khodagholi F, Shaerzadeh F, Ansari N, Sonboli A, Tusi SK. Cytotechnology. 2012; 64:403–419.
30. Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ, Taniguchi T. Brain Res. 1998; 780:260–269.
32. Kwon SH, Kim MJ, Ma SX, You IJ, Hwang JY, Oh JH, Kim SY, Kim HC, Lee SY, Jang CG. J Ethnopharmacol. 2012; 142:337–345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download