Abstract
The essential oils are fragrant products whose complex compositions are obtained from various parts of plants by dry or steam distillation. Plants with variable biological activities have been explored worldwide. The presence of a large number of phenols, terpenes and other aromatic compounds make essential oils more precise in their mode of action. Because of this, they are known to possess many biological activities like antimicrobial, antioxidant and anti-inflammatory etc. In this article, we will review the published literature summarizing the chemistry of essential oils and their important biological activities.
The essential oils are concentrated hydrophobic liquids containing volatile compounds obtained from plants. They are complex compounds which are characterized by a strong odor and are formed from various plants metabolites. They are limpid and soluble in lipid/organic solvents and possess density less than water. Essential oils are generally obtained by hydro or steam distillation. They were first developed by the Arabs in the Middle Ages and were known for their fragrances and various other medicina properties like antiseptic, bactericidal, virucidal and fungicidal (6). The first essential oil usage evidenced around 3000–2500 B.C. Egyptians were the first culture that used aromatic extracts for different purposes such as physical well-being, beauty care, culinary and spiritual uses. It is also believed that the extracts of essential oil have also been used in India and China at exactly the same time. Many essential oils are used for embalming, food preservation, analgesic, sedative, antimicrobial, anti-inflammatory, spasmolytic and localized anesthetic remedies. These characteristics of essential oils are responsible for their extensive use nowadays.
In nature, essential oils are having a great impact on plants generally by protecting them against herbivores by reducing their appetite. Several insects get attracted towards the fragrance of essential oils produced by plants and helps in the dispersion of seeds and pollens. The essential oils are abundantly present in plant organs like buds, stems, roots, seeds, fruits, flowers, leaves, twigs, wood or bark and thus stored in canals, secretory cells, cavities, epidermic cells or glandular trachoma. The extraction of essential oils is done from a range of aromatic plants which are usually grown in temperate to warm conditions.
There are a variety of methods used in the extraction of the essential oils, which includes the use of liquid carbon dioxide or microwaves, usually at low pressure or high-pressure distillation. The type of the products extracted also depends on the quantity, quality, soil composition, climate, plant organ, age and vegetative stage of plants (241). To attain the constant composition of essential oils, the need is to extract them under ideal conditions, from same plant organ, grown on same soil and from same season (41). Major commercialized essential oils are generally chemotyped by gas chromatographic and mass spectrometric analysis. Out of 3000 essential oils known till date, only 300 are important commercially with various applications in agronomic, pharmaceuticals, preservation of food, cosmetics, perfume and sanitary industries. For example, essential oils like d-limonene, d-carvone, and geranyl are having multiple uses in perfumes, soaps, creams, industrial solvents, as fragrances in household cleaning products and as flavoring additives in food. Other uses of essential oils include aromatherapy, massages with vegetable oil mixtures or in baths (2857). However, the growing interest in recent years towards natural products and essential oils, more focus is leading towards understanding their mode of biological action to identify their new applications in agriculture, environment, and human health. Many of the essential oils possess the potential to be used as alternatives or complementary natural compounds for improving human health (20).
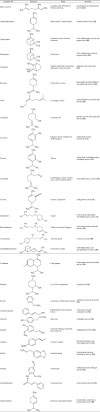
Essential oils are complex mixtures with different concentrations of various phytochemicals. They are generally characterized by some major components which are present at very high concentrations (20 – 70%) as compared to trace amounts of other components (Table 1). The biological properties of the essential oils are generally described by their major components. These components generally belong to two distinct groups of biosynthetic origin: terpenes/terpenoids and aromatic/aliphatic constituents, both further characterized by their molecular weights (2291447).
Terpenes are made up of 5-Carbon base (C5) units called isoprene. They are further classified on the basis of their structures and functions. Terpenoids are the terpenes that consist of oxygen. Terpenes are basically classified as monoterpenes (C10), sesquiterpenes (C15), hemiterpenes (C5), diterpenes (C20), triterpenes (C30) and tetraterpenes (C40) on the basis of the number of carbon atoms present. However, monoterpenes (C10) and sesquiterpenes (C15) comprise the major terpenes. Terpenes and terpenoids constitute 90% of essential oils with great variability in their structures. The different types of terpenes and terpenoids present in essential oils are- acyclic like myrcene, ocumene etc.; Monocyclic like terpinenes, phellandrenes, p-cimene etc.; bicyclic like sabinene, pinenes, camphene etc.; acyclic alcohols like geraniol, linalool, citronellol, lavandulol, nerol, etc., monocyclic alcohol like menthol, α-terpineol, carveol etc.; bicyclic alcohol like borneol, fenchol, thuyan-3- ol, chrysanthenol etc.; acyclic aldehydes like geranial, neral, citronellal etc.; acyclic ketones like tegetone etc., monocyclic ketones like carvone, piperitone, menthones etc., bicyclic ketones like fenchone, pinocarvone, ombellulone, thuyone etc.; acyclic esters like linalyl acetate or propionate, citronellyl acetate etc., monocyclic esters like menthyl or α-terpinyl acetate etc., bicyclic esters like isobornyl acetate etc.; ethers like menthofurane, 1,8-cineole etc; phenols like carvacrol, thymol etc.; peroxydes like ascaridole etc. The different enantiomeric forms are generally enriched in different plants; for example (−) linalool from coriander and (+) linalool from camphor trees, (+)-α-pinene from Pinus palustris and (−)-β-pinene from Pinuscaribae and Pinus pinaster etc. However, for some, one racemic form is more frequently present as compared to other e.g. (+)-citronellol is widespread, the form (+) is characteristic of Eucalyptus citriodora, the form (−) is common to the rose and geranium essential oils (24).
The aromatic derivatives of phenylpropane occur as often as terpenes. Although, the specific pathways for terpenes and phenylpropane derivatives synthesis are generally separated in plants, but may coexist with one main pathway over-taking the other e.g. cinnamon oil with cinnamaldehyde as major constituents. Aromatic compounds also show a lot of variabilities and can be grouped on the basis of functional groups for example Aldehyde like cinnamaldehyde; phenols like chavicol and eugenol; alcohol like cinnamic alcohol; methoxy derivatives like elemicine, anethole, estragole, and methyl eugenol; Methylene dioxy compounds like myristicine, apiole and safrole. These compounds are mainly enriched in spices and flavoring plants like cinnamon, anise, clove, fennel, sassafras, star anise, nutmeg, parsley, tarragon, and some botanical families (Apiaceae, Lamiaceae, Myrtaceae, Rutaceae). Glucosinolates or isothiocyanate derivatives present mainly in garlic and mustard oils are nitrogenous or sulfured components that are also characterized as secondary metabolites and are used in the manufacturing of grilled or roasted products (6).
Essential oils rich in the aromatic compound can also be classified on the basis of their aroma. For example floral aroma such as Neroli, Lavender and Jasmine; earthy such as Oakmoss, Vetiver and Patchouli; Citrus such as be orange, lemon and lime; herbaceous such as Marjoram, Rosemary and Basil; woodsy like pine, cedarwood, peppermint; minty like spearmint; oriental like ginger and Patchouli and spicy aroma like Nutmeg, clove and Cinnamon.
The diversity of chemical constituents present in essential oils makes them less precise in identifying their cellular targets (19). The essential oils are similar to typical lipophiles in terms of their permeability through the cell walls and other cytoplasmic membrane and thus can disrupt the complex composition and organization of fatty acids, phospholipids and polysaccharides to permeabilize them (2330345659). The mutagenic effects of essential oils had been evaluated using different analytical tests like SOS Chromotest with Escherichia coli, DNA Repair test with Bacillus subtilis, Ames test with Salmonella typhimurium or SMART assay with Drosophila melanogaster (612). The cytotoxic potential of essential oil depends on its chemical composition, which determines their ability to inhibit colony formation. There is variable cytotoxic response seen by different essential oils with different doses required to achieve 50% lethality. A study had shown that the cytotoxic effects of different essential oils on cells. They demonstrated that 50% lethality was achieved with 0.45 µL/mL for essential oil from Origanum compactum, 1.6 µL/mL for essential oil from Coriandrum sativum essential oil and >8 µL/mL for essential oils from Cinnamomum camphora, Artemisia herba-alba and Helichrysum italicum (7). Furthermore, the specific stage of cell growth also determines the sensitivity of cells to essential oils, as actively dividing cells are more sensitive and allow more effective penetration of essential oils. The cytotoxic potential of essential oils is generally attributed to the presence of aldehydes, alcohols, and phenols (1652). This property of essential oils is of great significance and can be exploited not only against certain human pathogens or parasites but also for preserving during longterm storage of agricultural and marine products.
Cancer is considered as the second largest death causing disease; with every year over six million lives were affected worldwide (39). Recently, there has been a remarkable increase in the usage of natural products to cure the cancer patients. Many drugs used to treat cancer like taxol, camptothecin, vincristine, and vinblastine are natural products (2943). Many other plants products had also been evaluated for their anticancer activity (60). During recent years, the herbal plants which produce essential oils have become the center of attention in the field of phytomedicine for cancer treatment (1758). The anticancer activities of essential oils are mainly due to free radicle production, membrane potential changes, overexpression of detoxification enzymes and oncogene modifications (38). They are also known to synergistically act along with conventional chemotherapy (51). Essential oils also play numerous roles in the analysis of melanomas, leukemias, glioblastomas and oral cancers (8).
Essential oils are attaining lot of importance in recent years as natural antimicrobial products. Multitudes of reports have evaluated the antimicrobial activity of essential oils against several microbial species (both bacteria and fungi) (26). A recent report had compared the antimicrobial activity of commercially available essential oils against microorganisms associated with skin infections i.e. Candida albicans, Malassezia spp., Propionibacterium acnes and Trichophyton spp. Among all, the best antimicrobial activity was demonstrated by Boswellia serrata essential oil (53). Another study had evaluated the antimicrobial efficiency of natural essential oils which can be used for root canal irrigation, using two activity tests i.e. contact and diffusion agar tests and all were found to be very effective (54). Minimum inhibitory concentration (MIC) assay was used to analyze the antimicrobial potential of 59 commercial essential oils generally recommended for dermatological conditions, out of them ten essential oils possess superior antimicrobial activity against all 13 tested micro-organisms. Further, determining their compositions by gas chromatography along with mass spectrometry identified eugenol as the main component responsible for the majority of essential oil's antimicrobial activity (44). Essential oil from six condiments used as preservatives in Brazil, i.e. basil, rosemary, marjoram, peppermint, thyme and anise were analyzed against Clostridium perfringens strain to combat foodborne diseases. All oils were demonstrated to possess bactericidal activity with MIC (minimum inhibitory concentration) for thyme: 1.25mg/mL, basil and marjoram: 5.0 mg/mL and rosemary, peppermint and anise: 10 mg/mL, for the. Only anise shows bacteriostatic activity (48).
Multitudes of studies have evaluated the anti-proliferative and pro-apoptotic activity of essential oils and shows promising results. These studies were generally conducted in vitro on various cancerous cell lines. Essential oil from Ocimum sanctum was found to inhibit proliferation and initiate apoptosis of MCF-7 cells (40). Nigella Sativa's essential oil was found to initiate pro-apoptotic signaling pathways in pancreatic ductal adenocarcinoma cell lines (49). The volatile oil of dried rhizome of Acorus tatarinowii Schott, a known herb to provide protection against cardiovascular diseases, was found to alter p53 status and induce apoptosis in human GBM (glioblastoma multiforme) cells (21). The essential oils extracted from both the wild-type as well as the cultivated Salvia Verbenaca plants, although possessing different composition were found to induce apoptosis in human melanoma cell line M14 (50).
The bioactive components of essential oil extracted from many medicinally important plants were known to provide protection against prolonged inflammation and improve human health. The anti-inflammatory properties of these plants are of immense importance for drug discovery process. The essential oil from Lindera erythrocarpa was found to be anti-inflammatory in RAW264 cells and down regulates LPS-induced pro-inflammatory mediator's production (35). Lavender essential oil was also found to inhibit inflammation through inhibition of TNF alpha (Tumor necrosis factor) and NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) in both murine brain and human umbilical vein endothelial cells (3). Linalool and cinnamaldehyde present in the leaf essential oil of indigenous cinnamon were found to be anti-inflammatory against endotoxin injected mice (37).
The essential oils are mentioned in Ayurveda for their therapeutic use to provide beneficial effects against different diseased states. Aromatherapy with essential oils is also known to improve health. Their property to naturally penetrate the skin is used to enhance transdermal drug delivery (25). The pharmaceutical and industrial uses of essential oil range from manufacturing of medicinal cosmetics, toothpaste, soaps and mouthwashes etc. Wild Turmeric (Curcuma aromatic Salisb) is extensively used as a constituent of many aromatic medicinal cosmetics in India (2755). Intensive research has been carried out nowadays to develop many different formulations with plant essential oils to increase their medicinal applications like microemulsions, nano-formulations etc (42).
Most of the human diseases are associated with the accumulation of reactive oxygen species produced either during the immune activity or triggered by factors such as pollution and smoke. Essential oils are known for their natural antioxidant activity. Extensive research have been carried out to analyze the antioxidative potential of different essential oils, generally analyzed by free radicle scavenging biochemical estimations like DPPH (α, α-diphenyl-β-picrylhydrazyl) and ABTs (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid). The antioxidant activity of essential oils depends on the presence of metabolites containing conjugated double bonds or phenolic groups (36). For example, essential oil from plants like Origanum tyttanthum, Thymus serpyllus and Mentha longifolia are enriched in phenolics like thymol and carvacrol, which are responsible for their antioxidant activity (18). Other functional groups like aldehyde, ketones, ethers and alcohols, those present in citronellal, geranial, menthone, geranial, cineole etc. which are the components of essential oils like cinnamon, clove, nutmeg, parsley, oregano and thyme also accounts for their antioxidant property (5). A recent report have shown that essential oil extracted from Salvia officinalis, Rosmarinus officinalis, Mentha piperita, Origanum vulgare, Allium sativum, Satureja montana, Foeniculum vulgare, Thymus vulgaris and Coriandrum sativum seeds being enriched in phytochemicals like thymol, linalool and carvacrol, were found to possess antioxidant activity as demonstrated by both ABTS and DPPH assays (46). The chloroform extract of Andrographis paniculata was found to have increased phenolic concentration and corresponding increased anti-oxidant activity (33).
Essential oils are the volatile compounds formed by the plants basically to provide protection by reducing the appetite of the herbivores eating them. All the plant parts like seeds, bud, flowers, roots, leaves etc. can be used for synthesizing essential oils. The ethnopharmacological properties of essential oils are mentioned in Ayurveda and used since ages for the treatment of different diseased states. They are known to possess biological activities like antimicrobial, antioxidant, cytotoxic and anticancer etc. These properties are the result of their chemical composition as different essential oils are enriched in different phytochemicals (Table 2). They are of immense economic importance and are used extensively in many pharmaceutical industries. However, more studies are required to elucidate their mechanism of action, dose required and toxicological effects to increase their potential uses.
Figures and Tables
References
1. Amorati R, Foti MC, Valgimigli L. J Agric Food Chem. 2013; 61:10835–10847.
2. Angioni A, Barra A, Coroneo V, Dessi S, Cabras P. J Agric Food Chem. 2006; 54:4364–4370.
3. Aoe M, Ueno-Iio T, Shibakura M, Shinohata R, Usui S, Arao Y, Ikeda S, Miyahara N. Acta Med Okayama. 2017; 71:493–503.
4. Arrebola E, Sivakumar D, Bacigalupo R, Korsten L. Crop Prot. 2010; 29:369–377.
6. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Food Chem toxicol. 2008; 46:446–475.
7. Bakkali F, Averbeck S, Averbeck D, Zhiri A, Idaomar M. Mutat Res. 2005; 585:1–13.
8. Bayala B, Bassole IH, Scifo R, Gnoula C, Morel L, Lobaccaro JM, Simpore J. Am J Cancer Res. 2014; 4:591–607.
9. Betts TJ. J Chromatogr A. 2001; 936:33–46.
11. Biondi D, Cianci P, Geraci C, Ruberto G, Piattelli M. Flavour Fragr J. 1993; 8:331–337.
12. Boran R, Ugur A. Pharm Biol. 2017; 55:402–405.
13. Bouchra C, Achouri M, Idrissi Hassani LM, Hmamouchi M. J Ethnopharmacol. 2003; 89:165–169.
14. Bowles EJ. Chemistry of aromatherapeutic oils. London: A&U Academic;2003.
15. Bozin B, Mimica-Dukic N, Simin N, Anackov G. J Agric Food Chem. 2006; 54:1822–1828.
16. Bruni R, Medici A, Andreotti E, Fantin C, Muzzoli M, Dehesa M, Romagnoli C, Sacchetti Sacchetti. Food Chem. 2003; 85:415–421.
17. Buckle J. Altern Ther Health Med. 1999; 5:42–51.
18. Caldefie-Chezet F, Guerry M, Chalchat JC, Fusillier C, Vasson MP, Guillot J. Free Radic Res. 2004; 38:805–811.
19. Carson CF, Mee BJ, Riley TV. Antimicrob Agents Chemother. 2002; 46:1914–1920.
20. Carson CF, Riley TV. Commun Dis Intell Q Rep. 2003; 27:S143–S146.
21. Chen L, Jiang Z, Ma H, Ning L, Chen H, Li L, Qi H. Sci Rep. 2016; 6:21148.
22. Croteau R, Kutchan TM, Lewis NG. Natural products (secondary metabolites). Biochemistry and molecular biology of plants. 2000. p. 1250–1318.
23. Di Pasqua R, Hoskins N, Betts G, Mauriello G. J Agric Food Chem. 2006; 54:2745–2749.
24. Djilani A. Nutrition, Well-Being and Health: The therapeutic benefits of essential oils. In Tech Open;2012. p. 155–178.
26. Essien EE, Thoma PS, Ascrizzi R, Setzer WN, Flamini G. Nat Prod Res. 2018; 1–4.
27. Gopinath H, Karthikeyan K. Indian J Dermatol Venereol Leprol. 2018; 84:16–21.
28. Hajhashemi V, Ghannadi A, Sharif B. J Ethnopharmacol. 2003; 89:67–67.
29. Heinrich MC, Maki RG, Corless CL, Antonescu CR, Fletcher JA, Fletcher CD, Huang X, Baum CM, Demetri GD. J Clin Oncol. 2006; 24:9502.
30. Helander IM, Alakomi HL, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, Von Wright A. J Agric Food Chem. 1998; 46:3590–3595.
31. Hussain AI, Anwar F, Hussain Sherazi. Food Chem. 2008; 108:986–995.
32. Janssen AM, Scheffer JJ, Baerheim Svendsen Baerheim Svendsen. Pharm Weekbl Sci. 1987; 9:193–197.
33. Kaur N, Gupta J. Int J Pharm Technol. 2017; 10:1271–1276.
34. Knobloch K, Pauli A, Iberl B, Weigand H, Weis N. J Essen Oil Res. 1989; 1:119–128.
36. Koh KJ, Pearce AL, Marshman G, Finlay-Jones JJ, Hart PH. Br J Dermatol. 2002; 147:1212–1217.
37. Lee SC, Wang SY, Li CC, Liu CT. J Food Drug Anal. 2018; 26:211–220.
39. Loizzo MR, Saab AM, Tundis R, Statti GA, Menichini F, Lampronti I, Gambari R, Cinatl J, Doerr HW. Chem Biodivers. 2008; 5:461–470.
40. Manaharan T, Thirugnanasampandan R, Jayakumar R, Kanthimathi MS, Ramya G, Ramnath MG. Pharmacogn Mag. 2016; 12:S327–S331.
41. Masotti V, Juteau F, Bessiere JM, Viano J. J Agric Food Chem. 2003; 51:7115–7115.
42. Natrajan D, Srinivasan S, Sundar K, Ravindran A. J Food Drug Anal. 2015; 23:560–568.
43. Newman DJ, Cragg GM. . J Nat Prod. 2007; 70:461–477.
44. Orchard A, Sandasi M, Kamatou G, Viljoen A, van Vuuren S. Chem Biodivers. 2017; 14:218.
45. Peana AT, D'Aquila PS, Panin F, Serra G, Pippia P, Moretti MD. Phytomedicine. 2002; 9:721–726.
46. Pellegrini M, Ricci A, Serio A, Chaves-López C, Mazzarrino G, D'Amato S, Lo Sterzo C, Paparella A. Foods. 2018; 7:pii: E19.
47. Pichersky E, Noel JP, Dudareva N. Science. 2006; 311:808–811.
48. Radaelli M, da Silva BP, Weidlich L, Hoehne L, Flach A, da Costa LA, Ethur EM. Braz J Microbiol. 2016; 47:424–430.
49. Relles D, Chipitsyna GI, Gong Q, Yeo CJ, Arafat HA. Adv Prev Med. 2016; 2016:1407840.
50. Russo A, Cardile V, Graziano AC, Formisano C, Rigano D, Canzoneri M, Bruno M, Senatore F. Nat Prod Res. 2015; 29:1630–1640.
51. Saab AM, Gambari R, Sacchetti G, Guerrini A, Lampronti I, Tacchini M, El Samrani, Medawar S, Makhlouf H, Tannoury M, Abboud J, Diab-Assaf M, Kijjoa A, Tundis R, Aoun J, Efferth T. Nat Prod Res. 2018; 32:1415–1427.
52. Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R. Food Chem. 2005; 91:621–632.
54. Shalaby A, Presneau N, Ye H, Halai D, Berisha F, Idowu B, Leithner A, Liegl B, Briggs TR, Bacsi K, Kindblom LG, Athanasou N, Amary MF, Hogendoorn PC, Tirabosco R, Flanagan AM. J Pathol. 2011; 223:336–346.
55. Sikha A, Harini A, Hegde Prakash LJ. Pharmacogn Phytochem. 2015; 3:1–4.
56. Sikkema J, De Bont JA, Poolman B. J Biol Chem. 1994; 269:8022–8028.
57. Silva NCC, Fernandes JA. J Venom Anim Toxins incl Trop Dis. 2010; 16:402–413.
58. Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J. J Ethnopharmacol. 2006; 103:99–102.
59. Ultee A, Bennik MH, Moezelaar R. Appl Environ Microbiol. 2002; 68:1561–1568.
60. Yousefzadeh MJ, Wyatt DW, Takata KI, Mu Y, Hensley SC, Tomida J, Bylund GO, Doublié S, Johansson E, Ramsden DA, McBride KM, Wood RD. PLoS Genet. 2014; 10:e1004654.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download