Abstract
Glycogen storage disease (GSD) IV is a rare autosomal recessive inherited disorder caused by mutations in the gene coding for glycogen branching enzyme leading to progressive liver disease. GSD IV is associated with mutations in GBE1, which encodes the glycogen branching enzyme. We report a case of GSD IV with rare homozygous mutations in the GBE1 gene (c.791G>A (p.Gly264Glu), which was successfully treated by liver transplantation.
Glycogen storage disease (GSD) IV is a rare inherited disorder characterized by the deficiency of α-1,4-glucan:α-1,4-glucan-6-transglucosylase (glycogen branching enzyme) [1]. This enzyme is involved in the formation of branches of the glycogen chains which makes glycogen more compact for storage. Deficiency in this enzyme results in accumulation of polyglucosan bodies in the liver causing progressive and fatal liver damage [23]. GSD IV is associated with mutations in GBE1, which encodes the glycogen branching enzyme. Several types of GBE1 mutations have been identified so far. In Korea, GSD IV was first reported in 1998 [4]. Herein, we present a case of GSD IV with rare homozygous mutations in the GBE1 gene.
A 4-month-old boy visited the outpatient department with abnormal liver function tests. There were no neurologic symptoms or signs. His body weight and height were 7 kg (25th–50th percentile) and 63.2 cm (25th–50th percentile), respectively. Vital signs were normal. On physical examination, the liver was hard and palpable by a handbreadth below the rib. Neurologic examination was normal. Complete blood count tests showed hemoglobin levels at 12.6 g/dL, a white blood cell count of 12,710/mm3, and platelets at 354,000/µL. Liver function and chemistry tests showed an aspartate aminotransferase (AST) level of 636 IU/L, alanine aminotransferase (ALT) of 262 IU/L, total bilirubin of 0.7 µg/dL, alkaline phosphatase of 300 IU/L, and creatine phosphokinase of 69 U/L . Coagulation profiles revealed a prothrombin time of 80%, an international normalized ratio (INR) of 1.15, and activated partial thrombin time of 46.2 seconds. Serological markers for hepatitis A virus, hepatitis B virus, hepatitis C virus, toxoplasma, rubella virus, cytomegalovirus, and herpes simplex virus were all negative. Abdominal ultrasound showed hepatosplenomegaly with periportal thickening and ascites. Transthoracic echocardiogram revealed no abnormal findings. A liver biopsy was performed, revealing micronodular cirrhosis with marked intracytoplasmic glycogen deposits (Fig. 1A, B). A liver biopsy was performed, revealing micronodular cirrhosis with marked intracytoplasmic glycogen deposits, and Periodic acid-Schiff (PAS)/D-PAS tests were positive/negative, respectively, which was consistent with the diagnosis of GSD (Fig. 1). Genetic testing for the G6PC gene (GSD Ia) was negative. Treatment with nightly cornstarch was initiated.
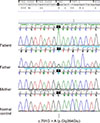
Six months later, the patient was hospitalized via the emergency department due to hematemesis. His mental status was drowsy. Vital signs revealed a blood pressure of 97/51 mmHg, heart rate of 153 beats/minute, respiratory rate of 32 breaths/minute, and body temperature of 36.3℃. On physical examination, his sclera were icteric, and his abdomen was distended with spider angiomas. His liver was hard and palpable at a handbreadth below the ribs and the spleen was palpable by two fingerbreadths. His hemoglobin was 6.2 g/dL, and coagulation profiles revealed a prothrombin time of 37%, INR of 2.09, and activated partial thrombin time of 54.4 seconds. Liver function tests showed an AST of 372 IU/L, ALT of 132 IU/L, total bilirubin of 4.5 µg/dL, direct bilirubin of 2.6 µg/dL, and ammonia of 94.7 µmol/L. Upper endoscopy revealed multiple grade III esophageal varices. Emergent ABO incompatible liver transplantation was conducted using the mother's liver as the donor. Molecular analysis of the GBE1 gene revealed homozygous variants of unknown significance, c.791G>A (p.Gly264Glu) (Fig. 2). GBE1 gene analysis in both parents revealed heterozygosity for the same mutation (Fig. 2). The patient recovered uneventfully and liver function tests were in the normal range at 14 days after transplantation. At the 12-month follow-up after transplantation, the patient was doing well without any complications.
GSD IV or Andersen disease is a rare autosomal recessive disorder caused by a deficiency in the glycogen branching enzyme, encoded by the GBE1 gene. GSD IV is estimated to occur in 1 in 600,000 to 800,000 individuals worldwide which accounts for approximately 3% of GSD cases [3]. There is significant molecular and clinical heterogeneity with several clinical variants having been described including the classic hepatic form, non-progressive hepatic form, neuromuscular neonatal form and adult polyglucosan body disease which presents with myopathy or cardiomyopathy symptoms. Although patients with the classic form of the disease are normal at birth, they are likely to develop failure to thrive and abdominal distention with hepatosplenomegaly in early childhood, followed by progressive liver failure and death by the age of 3 to 5 [5].
Liver transplantation is the only known treatment modality. The first successful liver transplantation for GSD IV was performed in 1984 [6]. To date, only 17 patients have been reported to receive liver transplants for this condition, and most did not develop cardiomyopathy or myopathy [7]. One patient died from cardiomyopathy after liver transplantation. Cardiomyopathy was not observed in our case. However, long-term follow up is necessary to prevent possible cardiac or neuromuscular complications.
The human GBE1 gene is located on chromosome 3p12 and consists of 16 exons [8]. GBE1 mutation analysis that has been performed in patients suggests a genotype-phenotype correlation, along with null mutations such as deletions, insertions, or nonsense mutations that are associated with a more severe clinical phenotype [5910]. More than 40 different mutations in the glycogen branching enzyme gene have been described. The variant found in our case was a missense mutation located on cDNA nucleotide 791, substituting the glycine codon at position 264 to a glutamate codon. This c.791G>A (p.Gly264Glu) variant has previously been reported in a Korean GSD IV case of a compound heterozygous with a previously known c.1571G>A (p.Gly264Glu) mutation [11]. However, to date there is no report regarding a homozygous mutation of c.791G>A (p.Gly264Glu) throughout databases and literatures. In order to predict the possible effects of new mutations on the structure and function of a human protein, this mutation was analyzed by PolyPhen-2 and Sorting Intolerant From Tolerant (SIFT) software. In silico analysis using PolyPhen-2 software showed that this mutation is probably damaging, with a score of 1.00, which is the highest possible score [12]. Analysis conducted with SIFT software also indicated that the mutation was likely deleterious [13].
In summary, we report a case of GSD IV with a rare homozygous variant in the GBE1 gene, in which the diagnosis was confirmed by genetic analyses in both parents.
Figures and Tables
References
1. Brown BI, Brown DH. Lack of an alpha-,4-glucan: alpha-, 4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. Proc Natl Acad Sci U S A. 1966; 56:725–729.

2. Andersen DH. Familial cirrhosis of the liver with storage of abnormal glycogen. Lab Invest. 1956; 5:11–20.
3. Glycogen storage disease type IV. Genetics Home Reference [Internet]. Bethesda (MD): US National Library of Medicine;cited 2017 Aug 10. Available from: https://ghr.nlm.nih.gov/condition/glycogen-storage-disease-type-iv.
4. Lee KY, Seo KH, Lee HK, Kim JW. Glycogen storage disease type IV: a case report. J Korean Med Sci. 1998; 13:211–214.

5. Bruno C, Cassandrini D, Assereto S, Akman HO, Minetti C, Di Mauro S. Neuromuscular forms of glycogen branching enzyme deficiency. Acta Myol. 2007; 26:75–78.
6. Selby R, Starzl TE, Yunis E, Todo S, Tzakis AG, Brown BI, et al. Liver transplantation for type I and type IV glycogen storage disease. Eur J Pediatr. 1993; 152:Suppl 1. S71–S76.

7. Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008; 12:137–145.

8. Thon VJ, Khalil M, Cannon JF. Isolation of human glycogen branching enzyme cDNAs by screening complementation in yeast. J Biol Chem. 1993; 268:7509–7513.

9. Moses SW, Parvari R. The variable presentations of glycogen storage disease type IV: a review of clinical, enzymatic and molecular studies. Curr Mol Med. 2002; 2:177–188.

10. Shin YS. Glycogen storage disease: clinical, biochemical, and molecular heterogeneity. Semin Pediatr Neurol. 2006; 13:115–120.

11. Ban HR, Kim KM, Jang JY, Kim GH, You HW, Kim K, et al. Living donor liver transplantation in a Korean child with glycogen storage disease type IV and a GBE1 mutation. Gut Liver. 2009; 3:60–63.

12. PolyPhen [Internet]. cited 2018 Mar 5. Available from: http://genetics.bwh.harvard.edu/pph.
13. Sorting Intolerant From Tolerant (SIFT) [Internet]. cited 2018 Mar 5. Available from: http://sift.jcvi.org.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download