Abstract
Accessory hepatic lobe is noted as and considered a rare disease in children. It can manifest with various symptoms and complications depending on the location, volume, type and position of the disease as presented on a child. The patient presented as a 14-month-old girl who was seen with a notable hepatosplenomegaly and portal hypertension. A diagnosis was made after taking an extensive medical history, observation and radiological examinations. The formal diagnosis was a prehepatic portal hypertension associated with accessory hepatic lobe.
Accessory hepatic lobe (AHL) is a rare congenital anomaly found in children, which is known to have resulted from an error in the formation of the endodermal caudal foregut during the embryogenetic process of a developing fetus, as noted most commonly in the third gestational week of a pregnancy. It may be completely separate, pedunculated or sessileor in characteristic, or else appear in the form of a lobe attached to normal hepatic tissue [1]. It is classified as type 1, a large lobe attached to the subphrenic or perihepatic region of the liver (>31 g); type 2, a lobe arising from around the right hepatic lobe or a wide base on the surface (11–30 g); and type 3, an entirely separate lobe frequently seen in the pelvis and the thorax or else in punctate form attached to the surface of the gallbladder or liver (<10 g) [123]. AHLs are generally asymptomatic conditions and are most often detected during surgery or autopsies. But it can be seen that sometimes these conditions can be presented with a torsion, hemorrhage, palpable mass in the upper right quadrant, acute or can be accompanied with chronic abdominal pain, and various symptoms associated with the compression and location of the AHL (such as dyspnea, chest pain, vomiting and solid organ obstruction) [4]. Here in, we reported a child with AHL that is associated with a prehepatic portal hypertension (PHT).
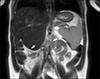
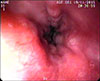
In this case, we noted a 14-month-old girl who presented with a preliminary diagnosis of hepatosplenomegaly. Upon review of her medical history, there were no pathological findings other than that a hepatosplenomegaly was present at the physical examination. Subsequently at the laboratory examination, hepatic enzymes, alpha-fetoprotein, alpha-1 antitrypsin, liver autoantibodies and coagulation parameters were normal, while the thrombocytopenia was observed at complete blood count (82×109/L). The portal vein Doppler ultrasonography revealed an incidence of hepatosplenomegaly and AHL, which is in the same echogenicity of the liver and approximately 5.5 cm in size extending to the right upper pole of the kidney, as noted with separate portal and hepatic veins. The main portal vein diameter was measured at 15 mm (normal lower limit <13 mm), and the flow rate is 30 cm/second (normal range 13–23 cm/second) [5]. Upon review of the magnetic resonance imaging of the liver, it was revealed that the AHL is 56×55 mm size extending inferiorly from the segment 5–6 level, with a separate portal and hepatic veins, and was noted to be compressing the right upper pole of the kidney on T2-weighted coronal image (Fig. 1). The endoscopic examination revealed grade 2 esophagial varices with three strands, and a notation that the varices were more evident when the patient lay on the left side during the endoscopy. The observed red spots were not visible during the review (Fig. 2). The patient was started on a program of propranolol (1 µg/kg/day) and followed with an endoscopic examination with a six months interval. During the follow-up, the patient underwent endoscopy and the grade of varices were noted as stable upon follow up. The noted thrombocyte levels were 104×109/L. The surgical treatment was planned in the case of a progression of varices grade or with the incidence of patient variceal bleeding.
The researchers have obtained a written consent form from the family in this case study.
The incidence of an AHL is a rare condition that generally develops as a result of embryonic heteroplasia in a patient. This rare condition is also thought to develop secondary to trauma or surgery in a patient. The condition may be associated with inheritance of an autosomal recessively transmitted gene. It has been hypothesized that AHL forms as a result of the embryonic liver curving outward during the intrauterine development stage of a patient's development, or of tunica muscularis recti development together with increased intra-abdominal pressure and liver growth [6].
The AHL is generally asymptomatic, and symptoms may vary depending on the localization and complication of the condition. Torsion is the most common and severe complication, but it may also present in a patient with acute/chronic abdominal pain, nausea, vomiting, chest pain, constipation, a palpable mass in the abdomen, swelling, dyspnea if located in the thorax and hemorrhage, and torsion or rupture if complications develop [7].
The complications of AHL may vary depending on the patient's age and location of the lobe. In infants it is generally associated with congenital acromphalus and biliary atresia. The incidence of pedunculated AHL is more risky because of complications, such as torsion, rupture or infraction. In addition, it may cause symptoms due to mechanical pressure placed on other organs or vascular structures, as in our case. A case of Riedel's lobe causing obstruction by compressing the stomach was reported in the literature. In that case, the patient received a physician performed cholecystectomy and fixing of the Riedel' lobe to the abdominal wall, with a simple suture, in an effort to manage the condition [8]. Additionally, a case of PHT developing due to external pressure caused by ectopic hepatic tissue was reported in an 8-year-old boy [9]. In another case, AHL was reported associated with vaginal varices and vaginal bleeding in a 25-year-old adult woman, while advanced tests revealed PHT and portal biliopathy developed due to extrinsic compression and occlusion of portal vein by the AHL. The patient in that case had perisplenic, perigastric collaterals varices and mild splenomegaly. Additionally noted was the cause of vaginal bleeding, which was seen as the incidence of dilate and tortuous vessels in the pelvis surrounding the uterus. The patient in that case was followed with symptomatic medical therapy and has not needed a surgical operation [10]. In our case, we believe that the PHT is related to both the compression of portal vein externally by AHL and increase portal vein blood flow by the AHL's own circulation. The patient is receiving medical treatment and controlled by intermittent endoscopy. Surgical treatment is performed when the patients are symptomatic or have complications as torsion, hemorrhage. There is no needed to treat patients with a sessile AHL connected to normal liver tissue [11]. In our patient, the AHL was identified coincidentally. The patient had only hepatosplenomegaly, and was considered stable on the follow up without any variceal bleeding.
In conclusion, AHL is considered a rare disease in children. It can manifest with various symptoms and complications depending on location, volume, type and position. There are very few reports in the literature of PHT developing secondary to the incidence of AHL compressing the portal vein. We want to share our experience with this case that AHL might be in the different diagnoses of prehepatic PHT.
Figures and Tables
References
1. Carrabetta S, Piombo A, Podestà R, Auriati L. Torsion and infarction of accessory liver lobe in young man. Surgery. 2009; 145:448–449.

2. Tancredi A, Cuttitta A, de Martino DG, Scaramuzzi R. Ectopic hepatic tissue misdiagnosed as a tumor of lung. Updates Surg. 2010; 62:121–123.

3. Mehta V, Arora J, Manik P, Suri RK, Rath G. Clinicoanatomical aspects of accessory fissures obscuring the normal hepatic morphology. Clin Ter. 2010; 161:259–260.
4. Glenisson M, Salloum C, Lim C, Lacaze L, Malek A, Enriquez A, et al. Accessory liver lobes: anatomical description and clinical implications. J Visc Surg. 2014; 151:451–455.

5. Chavhan GB, Parra DA, Mann A, Navarro OM. Normal Doppler spectral waveforms of major pediatric vessels: specific patterns. Radiographics. 2008; 28:691–706.

6. Pujari BD, Deodhare SG. Symptomatic accessory lobe of liver with a review of the literature. Postgrad Med J. 1976; 52:234–236.

7. Garba ES, Ameh EA. Isolated rupture of an accessory liver from blunt abdominal trauma in childhood. Pediatr Surg Int. 2002; 18:62–63.

8. Akbulut S, Cakabay B, Sevinc MM, Basak F. Gastric outlet obstruction caused by Riedel's lobe of the liver: a diagnostic and therapeutic challenge for surgeons. Hepatogastroenterology. 2011; 58:589–592.
9. Matley PJ, Rode H, Cywes S. Portal vein obstruction by ectopic liver tissue. J Pediatr Surg. 1989; 24:1163–1164.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download