Abstract
Drug-induced autoimmune hepatitis (DIAIH) is an increasingly recognized form of drug-induced liver injury that leads to a condition similar to idiopathic autoimmune hepatitis. A number of drugs have been associated with DIAIH, minocycline is one of the most well characterized. Minocycline is a semisynthetic tetracycline antibiotic used in the treatment of acne vulgaris. Minocycline-induced autoimmune hepatitis presents with serologic and histologic features similar to idiopathic autoimmune hepatitis. However, the natural history and outcomes of these two conditions differ significantly. The majority of patients with minocycline-induced autoimmune hepatitis experience complete resolution of symptoms after withdrawal of the medication. Some patients may require a short course of steroids and rarely use of an immunomodulator to achieve resolution of disease. Recurrence of symptoms is rare and typically only occurs with reintroduction of minocycline. It is important for primary care providers to consider minocycline-induced autoimmune hepatitis when liver injury develops during minocycline therapy.
Minocycline is a semisynthetic tetracycline antibiotic with broad antimicrobial activity that is active against Staphylococcus organisms and anaerobes [1]. It has a number of indications including treatment of urinary tract, ear, nose, and throat infections, pneumonia, and bronchitis [2]. It is also used for treatment of acne vulgaris and is one of the most widely prescribed systemic antibiotics for this indication. Minocycline has various characteristics that make it a good treatment option for acne vulgaris, including good oral absorption, enhanced tissue penetration (rapidly concentrated in sebaceous follicles), slow elimination, and improved patient compliance through once daily dosage. These benefits favor the use of minocycline over other anti-acne therapies [2]. Within this indication, it is often used for prolonged periods (months to years) in predominately young, otherwise healthy patients [3]. Its adverse effects have been well established and include transient headaches, nausea, light headedness, weakness, vertigo, and rash [1]. More serious adverse effects include autoimmune phenomena such as drug-induced lupus, a hypersensitivity-type reaction, vasculitis, serum sickness with arthritis, and autoimmune-like hepatitis [34]. We describe an adolescent male who developed minocycline-induced autoimmune hepatitis following minocycline therapy for acne vulgaris.
A previously healthy 17-year-old male presented to an emergency department with a three day history of nausea, poor appetite, pruritus, dark urine, and scleral icterus. He denied any acetaminophen or alcohol ingestion. There were no sick contacts. He had no prior history of hepatitis. There was no family history of liver disease or prior drug reactions. Vital signs were within normal limits and physical exam was significant for scleral icterus. No hepatosplenomegaly noted. Laboratory evaluation demonstrated significant transaminitis (aspartate transaminase [AST], 2,229 U/L; alanine transferase [ALT], 2,247 U/L) and conjugated hyperbilirubinemia (total bilirubin, 10.2 µg/dL; direct bilirubin, 5.9 µg/dL). Remainder of evaluation was normal which included: complete blood count, basic metabolic profile, and hepatitis panel (hepatitis A immunoglobulin [Ig] M, hepatitis B surface antigen, hepatitis B core antibody, hepatitis C antibody). He had been started on oral minocycline 100 µg once daily and topical clindamycin five months prior for treatment of acne. Both medications were discontinued and he was referred to pediatric gastroenterology clinic for further evaluation.
He was evaluated in pediatric gastroenterology clinic four days later at which time scleral icterus and pruritus persisted. Prior poor appetite and nausea had resolved but he did report a 3.2 kg weight loss. He denied abdominal pain, easy bruising/bleeding, lethargy, or acholic stools. Laboratory evaluation demonstrated a positive anti-smooth muscle antibody (1:160 titer) and elevated IgG level (1,766 µg/dL). Transaminitis (ALT, 3,227 U/L; AST, 3,547 U/L) and direct hyperbilirubinemia (total bilirubin, 21.8 µg/dL; direct bilirubin, 13.98 µg/dL) had worsened but he had normal hepatic synthetic function (international normalized ratio, 1.23; prothrombin time, 13.0 seconds). Other testing was normal including: antinuclear antibody (ANA), anti-liver-kidney-microsomal (anti-LKM) antibody, ceruloplasmin, infectious titers (Epstein-Barr virus, hepatitis A, hepatitis B, hepatitis C, human immunodeficiency virus, rapid plasma reagin), alpha-1-antitrypsin (A1AT) phenotype, total IgA level, tissue transglutaminase IgA, acetaminophen level, and urine drug screen.
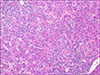
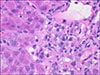
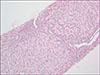
Outpatient management continued and an abdominal ultrasound with Doppler was completed; no hepatic or biliary abnormalities were noted but there was mild splenomegaly. A liver biopsy was scheduled and revealed prominent rosetting with increased plasma cells, slight increase in sinusoidal mature lymphocytes, and Stage 2 periportal fibrosis without bridging or cirrhotic nodularity. No eosinophils were present. There was no stainable iron, copper, or A1AT globules (Fig. 1, 2, 3). These histologic findings were consistent with moderate to severe acute-on-chronic hepatitis (overall grade 3 to 4) suggestive of autoimmune hepatitis. He was diagnosed with drug-induced autoimmune-like hepatitis secondary to minocycline based on these findings and overall clinical picture.
Transaminitis and direct hyperbilirubinemia persisted despite remaining off of minocycline. Transaminitis peaked with ALT of 3,422 U/L, AST of 3,909 U/L and direct bilirubin peaked at 17.54 µg/dL approximately two weeks after minocycline was discontinued. He was subsequently started on Prednisone with a taper over twelve weeks. He had a significant improvement in both transaminitis and direct hyperbilirubinemia within 24 hours of starting Prednisone and complete normalization of labs after 12 weeks. Hepatitis did not recur after discontinuation of prednisone. He did not undergo a repeat liver biopsy and was followed clinically with periodic monitoring of liver function tests.
The hepatotoxicity of tetracyclines has been recognized since 1952 [5]. While all tetracycline antibiotics are associated with adverse hepatic reactions, the risk associated with minocycline is higher than those within this drug class [2]. The mechanism by which minocycline induces liver injury is not known. Hepatitis may occur because minocycline is extensively metabolized in the liver while the primary route of elimination of other tetracyclines is by renal clearance [25]. Minocycline may also induce the upregulation or cause the production of specific antigens that activate an immune response leading to hepatitis in individuals with unique HLA characteristics [246]. A higher frequency of HLA-B*35:02 was found in patients with minocycline-induced autoimmune hepatitis than in the general population [7]. The exact mechanism by which HLA-B*35:02 increases risk of developing minocycline-induced autoimmune hepatitis is not known. Molecular docking data suggests that minocycline is capable of binding within the HLA-B*35:02 antigen binding cleft. This may alter the profile of peptide ligands normally bound to HLA-B*35:01 leading to presentation of novel cell surface antigens [7]. Another hypothesis is that minocycline metabolites bind HLA, forming complexes recognized by CD8+ cytotoxic T-lymphocytes [7]. Of note, risk alleles associated with idiopathic autoimmune hepatitis, HLA-DRB1*03:01 and HLA-DRB1*04:01, were not found to be risk factors for minocycline-induced autoimmune hepatitis [8].
Minocycline-induced autoimmune hepatitis generally occurs after a long period of exposure to the drug. The average onset is within 2 years of exposure, but may occur as soon as a few months [29]. Clinical features include fever, polyarthralgia, rash, anorexia, fatigue, nausea, abdominal pain, and jaundice [1]. The serological markers of the disease are similar to idiopathic autoimmune hepatitis. It is characterized by seropositivity for ANA, ASMA, and anti-LKM. Perinuclear anti-neutrophil cytoplasmic antibodies may also be present. Elevation in IgG level (>1.5 times the upper limit of normal) is also seen [19]. Histologic features of minocycline-induced autoimmune hepatitis are identical to autoimmune hepatitis. Chronic active hepatitis with periportal lymphoplasmacytic inflammation and piecemeal necrosis is typical [6]. Presence of eosinophils, which would be suggestive of a drug reaction, is rare [410]. Bridging fibrosis and cirrhosis are uncommon findings [9]. Minocycline-induced hepatitis occurs twice as frequently in women as it does in men. Onset of symptoms in women also occurs with a shorter duration of minocycline therapy when compared to men [9].
Symptoms and laboratory abnormalities typically resolve within 3 months after discontinuation of the medication [10]. Depending on severity of disease, use of oral corticosteroids such as prednisone may be required. Steroids should be continued for a multiple month course with an eventual taper. In certain cases, hepatic disease may be so severe as to require use of immunomodulators such as azathioprine. This additional therapy should be considered in cases with no improvement in transaminitis after both discontinuation of minocycline and at least 1-month use of corticosteroids. After treatment initiation, patients should be clinically followed for 1 year after normalization of lab values. Recurrence of disease does not typically occur unless the patient is re-challenged with minocycline [10].
While the overall risk of liver disease with minocycline is low, it is important that pediatricians and primary care providers are aware of the risk given how commonly the medication is prescribed for adolescent acne. At this time, no predictive factors have been described to help identify those individuals at greatest risk to develop minocycline-induced autoimmune hepatitis. Furthermore, no recommendations currently exist for surveillance during minocycline therapy [11]. It is essential to recognize the adverse effects of minocycline early as withdrawal of the medication results in rapid clinical improvement. Providers should consider the possibility of drug-induced autoimmune hepatitis when liver dysfunction develops during minocycline therapy.
Figures and Tables
References
1. Teitelbaum JE, Perez-Atayde AR, Cohen M, Bousvaros A, Jonas MM. Minocycline-related autoimmune hepatitis. Arch Pediatr Adolesc Med. 1998; 152:1132–1139.

2. Lawrenson RA, Seaman HE, Sundstrom A, Williams TJ, Farmer RDT. Liver damage associated with minocycline in use in acne. Drug Saf. 2000; 23:333–349.
3. Elkayam O, Yaron M, Caspi D. Minocycline-induced autoimmune syndromes: an overview. Semin Arthritis Rheum. 1999; 28:392–397.

4. Goldstein NS, Bayati N, Silverman AL, Gordon SC. Minocycline as a cause of drug-induced autoimmune hepatitis. Report of four cases and comparison with autoimmune hepatitis. Am J Clin Pathol. 2000; 114:591–598.
5. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009; 43:787–790.

6. Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010; 51:2040–2048.

7. Urban T, Nicoletti P, Chalasani N, Serrano J, Stolz A, Daly AK, et al. Minocycline hepatotoxicity: clinical characterization and identification of HLA-B/35:02 as a risk factor. J Hepatol. 2017; 67:137–144.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download