Abstract
Gastric hemangiomas are rare benign vascular tumors that can cause severe gastrointestinal system bleeding. We presented the case of a neonate with fresh bleeding and melena from the orogastric tube and detected gastric hemangioma in esophagogastroduodenoscopic examination. Propranolol is widely used in treatment of cutaneous hemangiomas and non-gastric gastrointestinal system hemangiomas. However, the surgical approach is preferred for treating gastric hemangiomas, and there are few reports of gastric hemangiomas associated with non-surgical treatment. Gastric hemorrhage decreased with antacid and somatostatin treatment. Propranolol treatment was initiated before the surgery decision. After three weeks of treatment, we observed regression in the hemangioma with endoscopic evaluation. During the course of treatment, the patient's gastrointestinal system bleeding did not recur, and there were no side effects associated with propranolol.
Gastrointestinal system (GIS) hemangiomas are benign vascular tumors. Although GIS hemangiomas may occur anywhere along the gastrointestinal tract, they usually occur in the small intestine followed by the colon and rectum. GIS hemangiomas in the stomach are rare. So far, 22 cases of gastric hemangioma have been reported in pediatric patients; of these, 13 were isolated cases [1234]. Esophagogastroduodenoscopy, angiography and computed tomography are valuable in diagnosis of gastric hemangiomas. In the case of gastric hemangiomas that can cause life-threatening hemorrhage with lesion ulceration, surgical involvement (total or partial gastrectomy, wedge excision) is the primary treatment option [56]. Propranolol is commonly used with corticosteroids to treat non-gastric GIS hemangiomas [7]. However, pharmacotherapy has often been performed in a small number of hemangioma patients with multiple lesions in the GIS, wherein surgical possibilities are limited [2348]. We report the case of a neonate with an isolated gastric hemangioma, presented with massive gastrointestinal bleeding, and the hemangioma shrunk with propranolol therapy after bleeding was controlled with antisecretory drugs. This is the first case of a neonate with gastric hemangioma treated with pharmacotherapeutic agents alone.
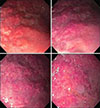
A female infant weighting of 2,220 g was born via vaginal route at 33 weeks of gestation. The neonate was admitted to the neonatal clinic at another centre due to a 24-hour history of preterm rupture of membranes and respiratory distress. The mother was age 26 years and this was her first pregnancy. On second postnatal day, fresh bleeding from the orogastric catheter and melena was detected and supportive treatment was initiated. Bleeding continued, and patient was administered erithrocyte suspension and referred to our hospital. Physical examination revealed that body weight was 2,200 g (50th–75th percentile), the length 45 cm (50th–75th percentile), and head circumference 32 cm (50th–75th percentile). The patient's axillary body temperature was 36.7℃, blood pressure was 65/35 mmHg, the pulse and respiratory rate 130 beats/minute and 50 beats/minute, respectively. The patient had phenotypic characteristics of Down syndrome, and other system examinations were normal. Results of the laboratory investigation were as follows: hemoglobin, 10.5 g/dL; leukocytes, 6.680/mm3; thrombocytes, 242.000/mm3. Bleeding time, coagulation and other biochemical test results were normal. GIS bleeding continued and somatostatin, omeprazol and ranitidin treatments were initiated. Enteral feeding was interrupted and abdominal graphy and portal vein Doppler yielded normal results. In the following days, gastric bleeding regressed with antacid therapy and somatostatin and the patient did not require transfusion. On day eight, the patient underwent esophagogastroduodenoscopy. Hemangioma was detected in the corpus and fundus of the stomach (Fig. 1). Biopsy specimen was not obtained as it may have caused additional bleeding. No cutaneous hemangioma was present and ultrasonographic examination revealed no hemangioma in the liver.
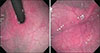
The patient was diagnosed with gastric hemangioma, and treatment with propranolol was initiated with 1 µg/kg per day divided into three doses and subsequently increased to 2 µg/kg per day the following days. Enteral feeding was increased. Somatostatin treatment was tapered and discontinued on day fourteen. No side effects (hypoglycemia, bradycardia, or respiratory distress) were observed in association with gastric bleeding and propranolol on subsequent days. On postnatal day twenty-two, patient was discharged with propranolol and antiacid treatment, and called for endoscopic examination three weeks later. Thecontrol endoscopy revealed marked regression in the size of the hemangioma (Fig. 2). Treatment was maintained to be completed at three months. Control endoscopy was to be performed after treatment is completed.
Hemangiomas may be observed in all organs, especially on the body surface as isolated or multiple lesions. However, visceral hemangiomas occur rarely except in the liver. Childhood GIS hemangiomas most commonly involve the small intestine and present with lower GIS bleeding. In more than half of these patients, cutaneous lesions are present, and some of these patients are diagnosed with posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta and other cardiac defects, and eye abnormalities (PHACE) syndrome [9]. Gastric hemangiomas, that occur more rarely, are frequently observed as isolated lesions. So far, 22 cases of gastric hemangioma have been reported in pediatric patients; of these, 13 were isolated cases [1234]. Four cases of hemangiomas were reported in the neonatal period, three of which were reported as isolated gastric hemangioma [24510].
GIS hemangiomas may occur in association with Maffucci syndrome, Klippel-Trénaunay syndrome, widespread neonatal hemangiamatosis and blue rubber-bleb nevus syndrome [11]. In our patient, chromosome analysis revealed presence of mozaic type trisomy 21 (46, XX/47, XX, +21), and hemangioma was not detected in other regions except the stomach. Interestingly, risk of vascular anomaly is lower in patients diagnosed with Down syndrome than in the general population. This is explained by increased expression of angiogenic factors [12].
As in this case, the most common symptom of gastric hemangioma is hematemesis, and is frequently accompanied by melena. More rarely, the patient may present with epigastric pain or symptoms of anemia secondary to chronic bleeding. Diagnostic tools that can be used irrespective of age and anatomic characteristics of the patients include imaging techniques such as computed tomography, magnetic resonance imaging, abdominal ultrasonography, scintigraphy and angiography. If it is thought that if bleeding originates from a lesion in stomach or duodenum, esophagogastroduodenescopic examination should be performed; if it originates from a focus in colon or rectum, colonoscopic examination is preferred. However, since most GIS hemangiomas are in the small intestine, esophagogastroduodenoscopy and colonoscopy may not be sufficient for imaging the lesion. In these patients, capsule endoscopy or scintigraphy are other options that can be performed for diagnosis [13]. In this case, contrast abdominal tomography and portal vein Doppler examinations were normal. A definitive diagnosis was made in detection of hemangioma by esophagogastroduodenoscopy in corpus and fundus of the stomach.
In treatment of GIS hemangioma, if there is a small, isolated lesion that causes mild symptoms, patients may be administered conservative treatments such as blood transfusion or iron supplementation [6]. First-line agents for symptomatic GIS hemangiomas are corticosteroids and propranolol, as in other infantile hemangiomas [1415]. Combined use of these agents may be performed in refractory cases. In addition, vincristine, interferon 1 alpha and other antiangiogenic drugs may be used [29]. For patients not responding to medical treatment, argon plasma coagulation, laser photocoagulation, sclerotherapy, band ligation or surgical resection are other treatment options [4816]. In a case series of 16 patients with gastrointestinal hemangioma, 8 diagnosed in neonatal period, 14 received pharmacological treatment. Of these patients, only two required intestinal resection as well as pharmacotherapy. However, none of these 14 patients had gastric hemangioma [9].
Treatment apporoach for gastric hemangiomas may differ from that for gastrointestinal hemangiomas. The clinical course of asymptomatic gastric hemangiomas is not completely known. However, in symptomatic gastric hemangiomas, that may be potentially life-threatening due to ulceration of the lesion, surgical resection is the preferred treatment option [15]. For this purpose, depending on the size and location of the lesion, excision of mass, antrectomy, and subtotal or total gastrectomy may be performed [17].
After gastric hemangioma is diagnosed, appropriate endoscopic and/or surgical treatment should be performed as soon as possible in to prevent serious complications that may potentially be life threatening. In this case, after bleeding was controlled with medical treatment, propranolol treatment was instituted before deciding on surgical intervention. In the weeks following propranolol treatment initiation, the patient was closely monitored in the clinic for GIS bleeding. There was no GIS bleeding and in control endoscopic examination performed three weeks later, the lesion had regressed and medical treatment continued. In a previous study of 22 gastric hemangioma cases reported in childhood, surgical intervention was performed in all cases except four [1234]. Presently, oral propranolol may be considered as first-line treatment in infantile hemangiomas [18]. Propranolol is also used commonly with corticosteroids in treatment of gastrointestinal hemangiomas [279]. However, few studies have reported treatment of gastric hemangiomas with methods other than surgical intervention, and these cases are usually with multiple lesions in which surgical options are limited. In a previous study, three patients were successfully treated with pharmacotherapy with additional argon plasma coagulation and electrocoagulation [248]. Similar to this case, a patient with multiple hemangiomas in the stomach and intestines successfully treated with propranolol was reported [3]. The most serious known adverse e ffects of propranolol are bradycardia, hypoglycemia and bronchospasm [19]. Hypoglycemia is common during propranolol therapy in the newborn and infantile period due to insufficient glycogen deposits and high rates of glucose consumption rates [20]. We did not observe side effects that may be associated with propranolol in our patient.
Unlike cutaneous lesions, it is not possible to directly visualise GIS hemangiomas to evaluate efficacy of drug treatment; therefore, endoscopic examination is required for evaluation of treatment. In this case, as regression was determined in control endoscopic examination, medical treatment was continued to be completed at three months. Repeat endoscopic examination was planned after completion of treatment.
In conclusion, we report a case of gastric hemangioma in a newborn. Although it is rare, hemangioma should be considered during differential diagnosis of cases with GIS bleeding. Unlike gastric hemangiomas, of which the most conservative and preferred treatment is pharmacotherapy, surgical is the preferred approach to treating gastric hemangiomas. We observed regression in hemangioma with propranolol therapy in our patient. We think that propranolol therapy must be performed before surgical intervention in selected cases under GIS bleeding control such as in our case.
Figures and Tables
References
1. Parolini F, Colusso M, Giannotti G, Cheli M, Alberti D. Gastric hemangiomas in children. Int J Gastroenterol Hepatol Transpl Nutr. 2016; 1:10–14.
2. Hansen LF, Wewer V, Pedersen SA, Matzen P, Paerregaard A. Severe blue rubber bleb nevus syndrome in a neonate. Eur J Pediatr Surg. 2009; 19:47–49.

3. Akcam M, Pirgon O, Salman H, Kockar C. Multiple gastrointestinal hemangiomatosis successfully treated with propranolol. J Pediatr Gastroenterol Nutr. 2015; 60:e16.

4. Lee YA, Chun P, Hwang EH, Lee YJ, Kim CW, Park JH. Gastric hemangioma treated with argon plasma coagulation in a newborn infant. Pediatr Gastroenterol Hepatol Nutr. 2017; 20:134–137.

6. Han EC, Kim SH, Kim HY, Jung SE, Park KW. Gastrointestinal hemangioma in childhood: a rare cause of gastrointestinal bleeding. Korean J Pediatr. 2014; 57:245–249.

7. Morris GA, Stratchko L, Sabri M. Intestinal hemangioma presenting as recurrent hematochezia in a 6-week-old male. J Pediatr Surg Case Rep. 2015; 3:280–282.

8. Khan K, Weisdorf-Schindele S. Gastric hemangiomas in an infant managed with argon plasma coagulation. Pediatr Endosurgery Innov Tech. 2003; 7:185–188.

9. Soukoulis IW, Liang MG, Fox VL, Mulliken JB, Alomari AI, Fishman SJ. Gastrointestinal infantile hemangioma: presentation and management. J Pediatr Gastroenterol Nutr. 2015; 61:415–420.
10. Nagaya M, Kato J, Niimi N, Tanaka S, Akiyoshi K, Tanaka T. Isolated cavernous hemangioma of the stomach in a neonate. J Pediatr Surg. 1998; 33:653–654.

11. Magnano A, Privitera A, Calogero G, Nanfito' L, Basile G, Sanfilippo G. Solitary hemangioma of the small intestine: an unusual cause of bleeding diagnosed at capsule endoscopy. J Pediatr Surg. 2005; 40:e25–e27.

12. Greene AK, Kim S, Rogers GF, Fishman SJ, Olsen BR, Mulliken JB. Risk of vascular anomalies with Down syndrome. Pediatrics. 2008; 121:e135–e140.

13. Kavin H, Berman J, Martin TL, Feldman A, Forsey-Koukol K. Successful wireless capsule endoscopy for a 2.5-year-old child: obscure gastrointestinal bleeding from mixed, juvenile, capillary hemangioma-angiomatosis of the jejunum. Pediatrics. 2006; 117:539–543.

14. Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013; 131:99–108.

15. Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: a retrospective comparative study. Pediatr Dermatol. 2011; 28:649–654.

16. Agnese M, Cipolletta L, Bianco MA, Quitadamo P, Miele E, Staiano A. Blue rubber bleb nevus syndrome. Acta Paediatr. 2010; 99:632–635.

17. Desa LA, Bridger J, Grace PA, Krausz T, Spencer J. Primary jejunoileal tumors: a review of 45 cases. World J Surg. 1991; 15:81–86.

18. Léauté-labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015; 372:735–746.

19. Horev A, Haim A, Zvulunov A. Propranolol induced hypoglycemia. Pediatr Endocrinol Rev. 2015; 12:308–310.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download