Abstract
Purpose
This study set out to evaluate the compliance to, and efficacy of oral supplementation, using a 1.5 kcal/mL or 1 kcal/mL sip feed, in children with mild to moderate malnutrition.
Methods
This was a parallel, randomized, controlled open-label trial in children aged 3 to 6 years with a weight for height Z (WHZ) score <−1 and ≥−3, who were randomized to receive a total of 600 kcal/day from either a 1.5 kcal/mL or a 1.0 kcal/mL pediatric sip feed for 28 days. Assessments included daily study product intake, body weight, tolerance and dietary intake from solid food.
Results
Of 110 children recruited, 98 (mean±standard deviation of age 49±7 months) completed the study. Both sip feeds were well tolerated, with high compliance (80±24% and 81±22% of prescribed volume in 1.5 kcal/mL and 1.0 kcal/mL groups respectively, p=0.79). Both study groups gained similar weight during the 28 days intervention period (0.42±0.40 kg in 1.5 kcal/mL group vs. 0.49±0.49 kg in 1.0 kcal/mL group, p=0.43). There were no significant differences between the groups in weight gain and in the change in WHZ score over the intervention period. Dietary analysis at the end of the study did not show replacement of solid food by the oral nutritional supplements.
Undernutrition is an important determinant of health status, as it is responsible for a large part of under-five child mortality and has long-term negative consequences [1]. Recent estimates reveal one out of ten children in Asia and Oceania is undernutrition [2]. According to National Basic Health Survey 2013, undernutrition in Indonesian under-five children was 12.1% [3].
Oral nutritional supplements (ONS) are useful to improve the dietary intake and nutritional status of children who are unable to meet their nutritional requirements with normal foods alone, despite dietary counselling to encourage the use of energy and protein rich foods.
Special pediatric oral nutrition supplements or sip feeds are available, with nutritional compositions, packaging designs and flavor varieties adapted to the needs of children. The choice of a suitable ONS for pediatric patients depends on the child's age, disease, nutritional requirements, oral intake, volume tolerance and taste preferences, as well as product availability and cost. Supplementation with high energy sip feeds may achieve a better compliance as the required volume of intake is reduced. However, several other factors are also known to influence compliance, including patient/family factors, and product related factors, such as taste, texture, and (lack of) variation. This study investigated the compliance and efficacy of ONS using 1.5 kcal/mL or 1 kcal/mL pediatric sip feeds in children with mild to moderate malnutrition.
Children with a weight for height Z (WHZ) score <−1 and ≥−3 were recruited from Posyandu (Integrated Service Post) and Pendidikan Anak Usia Dini (Early Age Child's Education) from 3 neighboring villages (Manggarai, Kenari, and Paseban, Central Jakarta) between March and May 2011. Children were deemed eligible for inclusion in the study if they were 3 to 6 years old, had a stable medical condition and could drink milk. Exclusion criterias were cow's milk allergy, galactosaemia, conditions needing a special diet, like major renal and hepatic dysfunction, major gastrointestinal intolerance (e.g., severe vomiting, severe diarrhoea), investigator's uncertainty about the willingness or ability of the child/carer to comply with the protocol requirements, as well as participation in any other study within two weeks prior to entry into the study. Written informed consent was obtained from the parents or caretakers.
The present study was a parallel, randomized, controlled open label trial comparing a 1.5 kcal/mL (NutriniDrink Multi Fibre®; Nutricia, Utrecht, the Netherlands; also marketed as Fortini MF®) to a 1.0 kcal/mL (NutriniDrink 1.0 Multi Fibre®, Nutricia) ONS in undernutrition children. Both ONS were nutritionally complete, milk-based, multi-fiber enriched, liquid pediatric sip feeds. Randomization was conducted using a computerized random number generator. This randomization was concealed in sequentially numbered opaque envelopes. It made impossible for the researcher to know the subject allocation without breaking the enveloped seal.
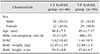
After written informed consent had been obtained from parents/caretakers, baseline characteristics (demographics, medical history, pre-existing conditions, medication use, the presence of edema, weight, and height) were recorded and subjects were randomized into one of two groups. The intervention group received 400 mL/day of the 1.5 kcal/mL ONS for a period of 4 weeks, and the control group received 600 mL/day of the 1.0 kcal/mL ONS for the same period. Composition of these study products is shown in Table 1. Both products were available in 200 mL bottles, in vanilla or chocolate flavor varieties. In both groups, the children were asked to choose their preferred flavor variety and were given the option to change the flavor variety during the intervention period. Every day, the investigator recorded the total volume of sip feed that had been consumed in both groups (using a measuring cylinder with 10 mL precision). Product appreciation questionnaires using a hedonic 7-point smiley face scale were completed by the children, with the help of their caregivers, after consuming the first serving of study product on day 1 and day 28.
During the intervention period, study product intake, body weight, tolerance and product appreciation were recorded for each group. Furthermore, total energy intake from food was assessed at baseline and on day 28 by a dietician, using the 24-hour dietary recall method. The occurrence of adverse events and medication use was recorded throughout the whole study period. The frequency and consistency of bowel movements were also recorded using Bristol Stool Chart (BCS). Diarrhea was defined as having at least 3 watery stools (BSC 6 or 7) per day.
The protocol was approved by the Ethics Committee of the Faculty of Medicine of University of Indonesia (No. 224/PT02.FK/43/N/2011).
Body weight of the children was measured using a Seca 767 digital column scale (Seca, Hamburg, Germany) at screening, one day before the start of the intervention, and on the day 8, 15, 22 and 29 of the intervention. The children wore minimal clothing when the body weight was measured. The height of the children was measured at baseline (using a Seca 206 measuring tape; Seca). At each time point, two measurements for height and weight were carried out. If the results from the two measurements differed, a third measurement was performed and the average result of the three measurements was recorded. The WHZ score of the children was assessed using the World Health Organization growth charts 2005. Mild to moderate malnutrition was defined as having a WHZ score <−1 and ≥−3.
To detect a 25% difference in the proportion of successful body weight increment between the two groups with 0.05 two-sided significance and 0.80 sensitivity, 49 subjects per group were required. Anticipating a dropout rate of 10%, a total number of 110 subjects was recruited.
Baseline characteristics of the children were reported using descriptive statistics. Data were analysed on an intention-to-treat basis with the SPSS statistical software package ver. 15.0 (SPSS Inc. Chicago, IL, USA). Data were compared between the two groups using the chi-square test for categorical data, Student's t-test for continuous data with normal distribution or Wilcoxon-nonparametric testing whenever appropriate. Statistical significance was set at p<0.05.
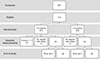
A total of 397 children were screened, of which 110 eligible subjects were recruited within a two-week period before the study. Fifty-seven subjects were allocated to the intervention group (receiving 1.5 kcal/mL ONS) and 53 subjects allocated to the control group (receiving 1.0 kcal/mL ONS). Five subjects were excluded from the study before the start of the intervention. Three of these children had gained weight after recruitment and their WHZ scores were above −1 on the day before the start of the intervention and two children were not willing to drink milk daily. During the intervention period, 7 subjects dropped out; 5 subjects in the 1.5 kcal/mL group and 2 subjects in the 1 kcal/mL group because of consent withdrawal, moving to other town and violations of the protocol (Fig. 1). The characteristics of study subjects are shown in Table 2. There were no significant differences in baseline characteristics between the two groups at the start of the study. There were more females in the control group (58.0%) than in the intervention group (45.8 %).
There were no significant differences between the two groups in total weight gain over the 4-week intervention period, daily weight gain, and the % of children who reached a WHZ score of >−1 (Table 3). There were also no significant differences in weekly weight gain between the two groups (data not shown). Subjects who achieved a WHZ score >−1 had a higher initial body weight at baseline and higher initial WHZ scores than subjects who did not. We observed similar compliance in both groups, with a product intake of 80±24% and 81±22% of prescribed volume in the 1.5 kcal/mL and 1.0 kcal/mL groups respectively (p=0.79). Both sip feeds were equally well appreciated; only 8 (16.7%) children in the intervention group and 7 children in the control group (14.0%) did not like the sip feeds (p=0.71).
There were no significant differences in total energy intake from food consumed between the two groups, both at baseline and day 28. Slightly lower energy intake from solid food after the intervention (−67.62±362.24 kcal in the intervention group and −100.88±391.58 kcal in control group) but this was not significantly different (p=0.67).
Both pediatric sip feeds were well tolerated, with no significant differences in stool characteristics and gastrointestinal symptoms between the groups. Stool frequency and consistency were similar, with most children having one bowel movement per day (0.94±0.25 vs. 0.97±0.26, p=0.64), with normal consistency (BCS 4-5).
Our study is the first to compare compliance and efficacy of ONS between standard energy (1.0 kcal/mL) and higher energy (1.5 kcal/mL) pediatric sip feeds in children without chronic diseases for the management of undernutrition. We carefully measured any left-overs feed daily to determine actual intake but we did not seek for the factors that contribute to compliance. Compliance is very important in oral nutritional interventions. Many factors influence compliance including healthcare setting and energy density. A systematic review of ONS in adult patients revealed higher compliance rate in community studies than in hospital setting (80.9% vs. 67.2%) [4]. In a retrospective study in pediatric outpatient clinics, compliance to pediatric ONS was reported to be 72% [5]. Compliance in our study is in line with these two studies, with product intakes of around 80% of the prescribed volume for each group.
There were no significant differences between the groups in terms of weight gain over the 4-week intervention period, or in the percentage of children reaching a WHZ score of >−1. By design, they received the same calorie supplementation, so we expected them to gain the same weight increments if they could drink the volume prescribed. In adult studies, volume, but not energy density, affects satiety. Higher energy density will increase energy intake [4]. Children tend to reduce their volume intake when presented with higher energy density especially in boys [67]. Our study did show that energy intake was equal between groups regardless the energy density of ONS.
We did not record daily food intake during the intervention period, but only at the baseline and at the end of the study. Analysis of total energy intake at day 28, did not show displacement of solid food by these ONS. The 24-hour recall method was used for assessing dietary energy intake, because of its simplicity and how easy it was to conduct. We are aware that this may not have been a very accurate method, in view of recall bias and day to day variations in food intake; however, other methods are also fraught with errors. The fact that we did not observe a significant decrease in energy intake from food is encouraging and is in line with earlier findings [8].
About one-third of the children in both groups achieved a WHZ >−1. These children had a higher initial WHZ than those who still had a WHZ <−1 after the 4-week intervention period. Our intervention period was quite short and this may indicate that those children with a lower WHZ may require longer nutritional intervention periods. In a study which investigated the efficacy of oral supplementation in undernutrition children with picky eater behavior, the oral supplement was given for 90 days [8]. It is to be expected that those children who did not sufficiently improve their nutritional status during the 4-week intervention period, would have reached a WHZ >−1 if the supplementation period had been longer. Interestingly, in our study, we demonstrated that children with milder forms of undernutrition can achieve catch up in weight in around 2 weeks of intervention. Thus, in clinical practice, it is important to tailor the design of intervention based on children initial condition.
Both sip feeds used in this study contained a multi-fiber mixture. Earlier studies in orally and tube fed children have shown that this multi-fiber mixture has a good safety and tolerance profile [910]. This study showed similar findings. There were no significant differences between the groups in terms of stool consistency and gastrointestinal symptoms. Stool frequency and consistency during the study were acceptable, with most children having one bowel movement per day with normal consistency.
Both 1.5 kcal/mL and 1 kcal/mL pediatric ONS are well appreciated and tolerated and are equally effective in promoting weight gain among children with mild to moderate undernutrition. Children with a milder form of malnutrition were able to achieve good nutritional status more quickly than those with more pronounced malnutrition.
ACKNOWLEDGEMENTS
This study was funded by Danone Research Grant (GA.PED.1.C.N.3.110309.A).We thank all children and their family for participating in the study and also all Posyandu Cadres, teachers of Pendidikan Anak Usia Dini.
References
1. Martins VJ, Toledo Florêncio TM, Grillo LP, do Carmo P Franco M, Martins PA, Clemente APG, et al. Long-lasting effects of undernutrition. Int J Environ Res Public Health. 2011; 8:1817–1846.

2. UNICEF-WHO-The World Bank. Levels and trends in child malnutrition. Joint Child Malnutrition Estimates, Key findings of the 2017 edition [Internet]. Washington DC: UNICEF-WHO-The World Bank;2017. 05. cited 2017 Dec 1. Available from: http://www.who.int/nutgrowthdb/jme_brochoure2017.pdf?ua=1.
3. Ministry of Health, Republic of Indonesia. Indonesia Health Profile 2013. Jakarta: Ministry of Health RI;2014. p. 114–117. ISBN 978-602-235-705-6.
4. Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clin Nutr. 2012; 31:293–312.

5. Smith C, Cawood AL, Jenkins C, Ralph A, Neville C, Lowdon J, et al. Review of use and compliance with oral nutritional supplements in dietetic pediatric outpatient clinics. A selection of abstracts presented at the British Dietetic Association Annual Conference, Manchester, UK: 15–17 September 2009. J Hum Nutr Diet. 2009; 22:581–608.
6. Evans S, Daly A, MacDonald A, Davies P, Booth IW. Impact of nutrient density of nocturnal enteral feeds on appetite: a prospective, randomised crossover study. Arch Dis Child. 2007; 92:602–607.

7. Kling SM, Roe LS, Sanchez CE, Rolls BJ. Does milk matter: Is children's intake affected by the type or amount of milk served at a meal? Appetite. 2016; 105:509–518.

8. Alarcon PA, Lin LH, Noche M Jr, Hernandez VC, Cimafranca L, Lam W, et al. Effect of oral supplementation on catch-up growth in picky eaters. Clin Pediatr (Phila). 2003; 42:209–217.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download