Abstract
Purpose
Studies on the physiology of the transposed stomach as an esophageal substitute in the form of a gastric pull-up or a gastric tube in children are limited. We conducted a study of motility and the pH of gastric esophageal substitutes using manometry and 24-hour pH measurements in 10 such patients.
Methods
Manometry and 24 hour pH studies were performed on 10 children aged 24 to 55 months who had undergone gastric esophageal replacement.
Results
Six gastric tubes (4, isoperistaltic; 2, reverse gastric tubes) and 4 gastric pull-ups were studied. Two gastric tubes and 4 gastric pull-ups were transhiatal. Four gastric tubes were retrosternal. The mean of the lowest pH at the midpoint of the substitute was 4.0 (range, 2.8–5.0) and in the stomach remaining below the diaphragm was 3.3 (range, 1.9–4.2). In both types of substitute, the difference between the peak and the nadir pH recorded in the intra-thoracic and the sub-diaphragmatic portion of the stomach was statistically significant (p<0.05), with the pH in the portion below the diaphragm being lower. The lowest pH values in the substitute and in the remnant stomach were noted mainly in the evening hours whereas the highest pH was noted mainly in the morning hours. All the cases showed a simultaneous rise in the intra-cavitatory pressure along the substitute while swallowing.
While “the native esophagus is the best esophagus”, esophageal replacement becomes necessary in certain conditions. The stomach has become a popular substitute in recent decades. However, preparation of the stomach as an esophageal substitute is associated with substantial alteration in its blood supply, capacity, parietal cell mass, innervation, and shape (due to stretching); and relocation of the organ partly or completely in the intrathoracic position has its own implications. This surgical procedure has effects on gastric secretion, motility and gastric emptying [1].
The physiology of the transposed stomach has been studied mainly in adults and the results are still debated [2]. Many case reports and series of gastric transpositions and gastric tube in children address the residual gastric motility after gastric transposition. However, studies of manometry and pH of gastric esophageal substitutes in children are relatively sparse. This study was designed to evaluate the motility of the gastric tube and the pulled-up stomach by manometry and 24-hour pH study in gastric esophageal substitutes.
This study was an observational evaluation of 10 patients who had undergone an esophageal replacement. These patients were investigated from 9 months to 43 months after the operation. The average time since surgery at which the patients were investigated was 20.9 months. All the patients were subjected to a manometry and a 24-hour pH study.
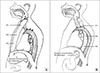
Manometry was performed using a 16 channeled high resolution system (which uses software to analyze the manometry trace) for 30 minutes. This system includes 16 channel water perfusion high resolution manometry with spatiotemporal analysis plot. The pressure profiles of the upper, mid and lower portion of the transposed substitute were measured through multiple ports of a polyurethane 16-channel catheter. Ports were placed at a distance of 3 cm along the length of the substitute and the distance decreased to 1 cm at the lower esophageal sphincter (LES) and in the remaining part of native stomach below the diaphragm. These positions were labeled P1 (9 cm above the level of the diaphragm), P2 (6 cm above the level of the diaphragm), and P3 (3 cm above the level of the diaphragm) (Fig. 1). The numbers of ports used varied according to length of substitute and the part of the stomach remaining below the diaphragm. The catheter was connected to transducers; a continuous flow of distilled water was maintained through the catheter channels at a rate of 0.5 mL/min.
The catheter was inserted through the nostril and advanced until the lowermost port of the catheter corresponded to the antrum of the stomach. Intraluminal pressures were measured at rest and during swallowing. Children were given only water to swallow. Pressure tracings were seen on the monitor with data acquisition at 25 Hz.
Manometry was performed without sedation in almost all the patients except for a few, in whom injection midazolam 0.01µg/kg was given. It has been proven that midazolam has minimal or no influence on pressure measurements [3].
Twenty-four hour pH studies were carried out using an antimony electrode with an external reference electrode, connected to a recording device, i.e., a gastrograph. The system was calibrated for pH 4 and pH 7 for every patient. The antimony electrode had two sensors; the lower sensor was placed in the intra abdominal portion of the stomach, and the upper sensor was placed at the midpoint of the pulled up stomach or reconstructed gastric tube (the midpoint from the diaphragm to the cricopharynx). The position of the sensors was confirmed using a C-arm.
The patients were taught to mark events such as feeding, crying and variation in position (upright and supine). The child was allowed to carry out routine activities and feed normally.
The gastrograph records the pH at the midpoint of the substitute and in the stomach remaining below the diaphragm every 4 seconds and stores the data. An event marker incorporated into the gastrograph marks the events. The data is analyzed by a computer.
The lowest pH, the highest pH, and the relationship between pH and food, both in the esophageal substitute and in the stomach remaining intra-abdominally were studied. Pressure changes in the substitute were also studied in resting and swallowing phases. Of the 10 patients studied, 6 were boys and 4 were girls. The age range was 2 years to 4 years 7 months with 3 patients each in the 2 to 3 years and 3 to 4 year age groups and 4 patients in the 4 to 5 year age group (Table 1). The patients were investigated 9 months to 43 months after the operation. The average time since surgery at which the patients were investigated was 20.9 months. Six patients had a gastric tube esophagoplasty of which 4 were isoperistaltic and 2 were reverse gastric tubes. Four patients had a gastric pull-up. Two gastric tubes and 4 gastric pull-ups were done by the transhiatal route. The remaining 4 gastric tubes were brought up through the retrosternal approach.
The lowest pH at the midpoint of the substitute ranged from 2.8 to 5.0 (mean of 4) and in the stomach remaining below the diaphragm ranged from 1.9 to 4.2 (mean of 3.3). The highest pH values recorded at the midpoint of the gastric tubes were 8.0, 7.8, 7.2, 7.8, 8.1, and 7.9 with a standard deviation (SD) of 0.316. For gastric pull-ups, the highest pH at the midpoint of the thoracic portion were 7.2, 7.9, 8.4, and 7.7 with an SD of 0.496; the lowest pH were 2.8, 3, 5, and 3.7 with an SD of 0.99. The highest pH in the stomach remaining below the diaphragm in all the 10 patients was between 7 and 8. In patients with gastric tube esophagoplasty, the highest pH in the portion of the stomach below the diaphragm were 7.3, 7.6, 7.5, 7.3, 7.4, and 7.6 with an SD of 0.1378. The lowest pH in this portion were 4.0, 2.8, 3.4, 3.9, 4.1, and 3.4 with an SD of 0.493. In case of gastric pull-ups, the highest pH in the portion of the stomach below the diaphragm were 7.3, 7.5, 7.3, and 7.1 with an SD of 0.163 and the lowest pH in this portion was 1.9, 2.2, 4.2, and 3.0 with an SD of 1.02.
In both types of substitute, the difference between the peak and the nadir pH recorded in the intra-thoracic and the sub diaphragmatic portion of the stomach was statistically significant (p<0.05; paired t-test), with the pH in the portion below the diaphragm being lower.
The average peak pH in the intrathoracic gastric tube was 7.8 and the average nadir pH was 4.3. The average peak pH in the intrathoracic gastric pull-ups was 7.8 and the average nadir pH was 3.62. In the stomach remaining below the diaphragm, the average peak pH in the case of gastric tube esophagoplasty was 7.45 and the average trough pH was 3.6; whereas in the case of gastric pull-ups, the average peak pH in the stomach remaining below the diaphragm was 7.3 and the average trough pH was 2.8.
The lowest pH values in the substitutes and in the remnant stomachs were noted mainly in the evening hours whereas the highest pH was noted mainly in the morning hours (Table 2).
In a period of 24 hours, at the midpoint of the substitute, a pH of more than 6 was noted 70% of the time. During 26% of the time, the pH was between 4 and 6 and only during 4% of the time did the pH dip below 4. In the stomach remaining below the diaphragm, during 68% of the time the pH was between 4 and 6 and about 18% of the time the pH was in between 2 and 4.
In supine position, the pH values at the midpoint of the gastric tube were 4.3, 7.6, 4.9, 5.2, and 4.8 with an average of 5.3 and an SD of 1.6. In patients with gastric pull-up, the pH values at the midpoint of the thoracic portion of the stomach were 3.9, 4.5, 5.8, and 5.1 with an average of 4.83 and an SD of 0.8.
The relationship between food and pH was also studied. The pH at the midpoint of the substitute and in the stomach remaining below the diaphragm was measured 30 minutes and 2 hours after a meal. The pH at the midpoint of the gastric tube substitute 30 minutes after a meal was 4.91 (mean) with an SD of 1.14. The pH at the same site 2 hours after a meal was 4.6 (mean) with an SD of 0.5. The pH at the midpoint of the gastric pull-up substitute 30 minutes after a meal was 4.4 (average) with an SD of 1.1. Two hours after a meal, the pH at the midpoint of the gastric pull-up substitute averaged 4.45 with an SD of 0.9.
The pH in the stomach remaining below the diaphragm in the cases of gastric tube esophagoplasty 30 minutes after a meal was 4.58 (average) with an SD of 1.22 and 2 hours after a meal was 4.63 with an SD of 1.14.
In the case of gastric pull-ups, the pH 30 minutes after a meal in the portion of the stomach below the diaphragm was 3.57 on average, with an SD of 0.62 whereas 2 hours after a meal, the pH in this portion of the stomach was 3.35 (average) with an SD of 0.77 (Table 3).
The average resting pressure in the patients with a gastric tube esophageal substitute throughout the substitute (i.e., average of P1, P2, and P3) was 1.358 mmHg and at the level of the diaphragm (i.e., LES) it was 3.65 mmHg.
The average resting pressure of the stomach remaining below the diaphragm was 2.06 mmHg.
In patients with gastric pull-up, the average resting pressure throughout the substitute (i.e., average of P1, P2, and P3) was 1.21 mmHg and at the level of the diaphragm (i.e., LES) it was 1.945 mmHg. The average resting pressure of the stomach remaining below the diaphragm was 1.845 mmHg.
The average peak pressure of the gastric tube throughout the substitute (average of P1, P2, and P3) was 10.64 mmHg and the pressure at the level of diaphragm was 19.66 mmHg.
The average peak pressure of the stomach remaining below the diaphragm was 7.45 mmHg.
The average peak pressure of the gastric pull-up at P1, P2, and P3 was 9.293 mmHg. The average peak pressure at the level of the diaphragm was 11.84 mmHg. The average peak pressure of the stomach remaining below the diaphragm was 7.007 mmHg.
Of the many procedures described for esophageal replacement, gastric transposition has been considered the first choice [14]. According to a study by Hirschl and Yardeni [5] this procedure has few short term complications; however there are some long term complications [4], including the concern for Barret's esophagus in the proximal esophageal remnant. Such outcomes would be dependent on the substititute's ability to clear secretions and boluses, as well as its ability to maintain a physiological pH. Gupta et al. [2] performed a manometric evaluation of the intrathoracic stomach after gastric transposition in 18 children but the pH was not studied in that series. Hence, our study was designed to evaluate the motility of the gastric tube and the whole stomach by Manometry and 24-hour pH study of of the esophageal.
Gastric transposition in any form such as gastric tube (either reverse gastric tube or isoperistaltic gastric tube) or gastric pull-up cannot function fully as a native esophagus. Factors such as disruption in the neuronal plexus, alteration in blood supply and ectopic position of the stomach definitely alter the pressure waves generated in the substitute. The exact motor behaviour of the stomach as an esophageal substitute is not well understood. A few studies based on motor response suggest that it merely acts as an inert organ. However, recent follow-up studies question this concept and there is increasing evidence that the transposed stomach recovers some motor activity and may even generate complete migrating motor complexes [6]. Studies using electrical impedance tomography and surface electrography to study gastric emptying following gastric transposition have reported that the transposed stomach does not behave as an inert conduit but retains its reservoir function with an extremely irregular emptying pattern [789].
Our study found a simultaneous rise in the intra-cavitatory pressure at P1, P2, P3 during a swallow in all the cases, suggesting simultaneous contraction of the whole substitute. There was no propulsive progressive propagated peristaltic wave in any of the patients studied. Mass contractions were observed in all the cases. Our finding of mass contractions in response to swallowing indicate that there probably is functional integrity of the neuronal plexuses and that while the waves may not appear progressive there is definite evidence of swallow related motor activity in the stomach. Whether this mass contraction ultimately will evolve into a peristalsis that effectively clears gastric secretions away from the esophageal remnant remains to be seen and long term follow up of our patients may provide the answer to this.
All the patients in our study were in the preschool age group. According to Nagita et al. [10], the normal pH of the stomach in the preschool age group is 1 to 3. In our study, the average pH of substitute as well as the stomach in both groups is higher as compared to normal [10].
At the midpoint of the substitute, the pH was more than 6.0 about 70% of the time. During 26% of the time the pH was between 4 and 6 and during 4% of the time the pH was less than 4. In the stomach (below the diaphragm), during 68% of time the pH was between 4 and 6 and about 18% of time the pH was in between 2 to 4.
The lowest pH at the midpoint of the substitute averaged 4.3 in gastric tubes and 3.6 in gastric pull-ups. The lowest pH in the stomach remaining below the diaphragm averaged 3.6 in gastric tubes and 2.8 in gastric pull-ups. The lowest pH in the substitute and the stomach was noted mainly in the evening hours. The highest pH at the midpoint of the substitute and the stomach remaining below the diaphragm was noted mainly in the morning hours. These findings suggest that normal gastric circadian rhythm is maintained in the substitute. It could either be due to reflux of gastric juice into the substitute from the stomach remaining below the diaphragm or due to gastric secretion from the substitute. It can also be attributed to circadian autonomic nervous outflow.
Gutschow et al. [11] found the normal gastric pH profile in 32.3% of patients who were operated within 1 year, in 81.5% of those operated between 1 and 3 years previously and in 97.6% of those patients operated more than 3 years before the study.
The initial reduction of acid secretion in the transposed stomach could be because of vagal denervation, alterations in the blood supply of the stomach, or decreased stomach capacity. In addition to these factors, a decrease in the number of parietal cells is also responsible for decrease in acid production in the gastric tube [1].
Intraluminal pH is gradually restored. This is probably similar to the increase in acid output with time in adult patients vagotomised for duodenal ulcer disease [9].
Gutschow et al. [11] discussed the cause of progressive acidification in the denervated stomach as an esophageal substitute and argued that “the vagus nerve fibers have no direct connection with the sub mucosal plexus but terminate on the myenteric plexus within the lesser curvature, so that vagal inputs reach the submucous plexus only through interconnective path ways originating from the myenteric ganglia.” However, the enteric nervous system within the gastric wall contains programs that organize both secretory and motor functions of the stomach; this ‘local brain’ can progressively function without any driving input from the central nervous system. Post-vagotomy ultra structural changes in neurons within the gastric wall and in parietal cells are reversible, while acid secretion increases towards preoperative values. Better recovery of the secretory than the motor function of the denervated stomach probably reflects easier self-reorganization of the sub mucous plexus because of a less complex architecture than the myenteric plexus. Moreover, unlike those in the myenteric plexus, nervous ganglia in the submucous plexus have not been shown to be concentrated within the lesser curvature, minimizing the role of resection of the lesser curvature in the recovery of a normal intraluminal pH profile.
Patterson et al. [12] in their longitudinal study found statistically significant differences in the electrical control activity pattern at birth and that seen at the age of six months, suggesting that neonatal electrical control activity undergoes a maturation process. This is an interesting phenomenon as it may follow in the transposed stomach and could be the cause of increased acidity in the substitute over time.
The average pH at the midpoint of the gastric tube 30 minutes after a meal was 4.9 and 2 hours after a meal was 4.6. In the portion of the stomach below the diaphragm after gastric tube construction, the pH 30 minutes after a meal was 4.58 and 2 hours after a meal was 4.63. In gastric pull-ups the pH 30 minutes after a meal as well as 2 hours after a meal was 4.45. According to the study by Nagita et al. [10] postprandial peak intragastric pH values vary with age from 7 in neonates to about 4 in adolescents. Postprandial increases in intragastric pH lasted approximately 3 hours in children less than 10 years of age and lasted from 30 minutes to 2 hours in subjects over 10 years of age.
The salient findings of the present study can be summarized as follows: although there was no propulsive progressive propagated peristaltic wave in any of the patients studied, mass contractions were observed in all cases. There was no late mass contraction in response to swallow in any of the patients. A rise in pressure in the substitute was noted during swallowing suggesting that the substitute may be able to clear contents.
A circadian rhythm is maintained in the substitute. These findings need to be evaluated in a larger cohort over a longer period of time. Our finding that the substitute contracts during swallowing could have a bearing on the long term use of proton pump inhibitors and prokinetic agents in this group.
Figures and Tables
ACKNOWLWDGEMENTS
The authors express their sincere gratitude to Dr. Jayashree Mondkar, Dean, Lokmanya Tilak Municipal Medical College and Lokmanya Tilak Municipal General Hospital.
References
1. Hölscher AH, Voit H, Buttermann G, Siewert JR. Function of intrathoracic stomach as esophageal replacement. World J Surg. 1988; 12:835–844.
2. Gupta DK, Charles AR, Srinivas M. Manometric evaluation of the intrathoracic stomach after gastric transposition in children. Pediatr Surg Int. 2004; 415–418.

3. Fung KP, Math MV, Ho CO, Yap KM. Midazolam as a sedative in esophageal manometry: a study of the effect on esophageal motility. J Pediatr Gastroenterol Nutr. 1992; 15:85–88.
4. Davenport M, Hosie GP, Tasker RC, Gordon I, Kiely EM, Spitz L. Long term effects of gastric transposition in children: a physiological study. J Pediatr Surg. 1996; 31:588–593.

5. Hirschl RB, Yardeni D, Oldham K, Sherman N, Siplovich L, Gross E, et al. Gastric transposition for esophageal replacement in children: experience with 41 consecutive cases with special emphasis on esophageal atresia. Ann Surg. 2002; 236:531–539. discussion 539-41.
6. Collard JM, Romagnoli R, Otte JB, Kestens PJ. The denervated stomach as an esophageal substitute is a contractile organ. Ann Surg. 1998; 227:33–39.

7. Powe JE, Casson AG, Inculet RI, Laurin N, Finley RJ. Functional evaluation of gastric interposition following total esophagectomy. In : Proceeding of the 37th Anuual Meeting of Nuclear Medicine; 1990. 31:p. 775. (abstract).
8. Ravelli AM, Spitz L, Milla PJ. Gastric emptying in children with gastric transposition. J Pediatr Gastroenterol Nutr. 1994; 19:403–409.

9. Romeo G, Giovinetto A, Sanfilippo G, Calì R, Catania G, Basile F. Follow-up study in 402 patients after parietal cell vagotomy for duodenal ulcer. Int Surg. 1981; 66:303–306.
10. Nagita A, Amemoto K, Yoden A, Aoki S, Sakaguchi M, Ahida K, et al. Diurnal variation in intragastric pH in children with and without peptic ulcers. Pediatr Res. 1996; 40:528–532.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download