Abstract
Helicobacter pylori plays an important role in the pathogenesis of chronic gastritis, peptic ulcer disease, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma. In Korea, the guidelines for the diagnosis and treatment of H. pylori infection in adults were revised in 2013. The European Helicobacter and Microbiota Study Group and Consensus panel released the fifth edition of the Maastricht Consensus Report for the management of H. pylori infection in 2015, and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition and the North American Society of Paediatric Gastroenterology, Hepatology and Nutrition released the updated joint guidelines for children and adolescents in 2016. Considering these recommendations and recent progress in our research and that of other research teams, this study aimed to discuss the diagnostic strategies for H. pylori infection in children and adolescents.
Helicobacter pylori plays an important role in the pathogenesis of chronic gastritis, peptic ulcer disease, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma. The seroprevalence rates of anti-CagA antibodies among Koreans in 1988 to 1989 were as follows: 25% at 2 to 4 years of age, 50% at 5 years of age, and 80% to 90% after 7 years of age [1]; the same kind of study conducted in 2014 to 2015 revealed 15% at 5 to 14 years of age, 20% at 15 to 19 years of age, 30% at 20 to 29 years of age and over 80% after 30 years of age (unpublished observation). H. pylori infection begins at infancy and is established before 5 years of age, and non-infected persons have a very limited chance of acquiring the infection [2]. H. pylori, once acquired during childhood, is rarely eliminated without specific treatment and the infected individuals live with it all their lives [3].
In Korea, the guidelines for the diagnosis and treatment of H. pylori infection in adults were revised in 2013 (Korea Adult Guidelines) [4] and have been used in routine clinical practice up until today. Recently, the European Helicobacter and Microbiota Study Group and Consensus panel released the fifth edition of the Maastricht Consensus Report for the management of H. pylori infection in 2015 (Maastricht Consensus V) [5], while the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the North American Society of Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) released the updated joint ESPGHAN/NASPGHAN guidelines for children and adolescents in 2016 (ESPGHAN/NASPGHAN guidelines) [6]. However, not only is it inappropriate to apply the diagnosis and treatment strategies for H. pylori infection established in adults to children and adolescents, but it is also inappropriate to directly apply the guidelines developed in Europe and North America for children and adolescents to those living in Korea where the epidemiology of H. pylori is quite different. To date, there is no established Korean consensus on the diagnosis and treatment of H. pylori infection in pediatric patients.
Considering these recommendations and recent progress in our research and other research teams, this study aimed to discuss the diagnostic strategies for H. pylori infection in children and adolescents.
The indications for the endoscopic diagnosis and treatment of H. pylori infection in the Korean Adult Guidelines are (i) peptic ulcer disease including scar, (ii) low-grade gastric MALT lymphoma, (iii) resection of early gastric cancer, (iv) chronic atrophic gastritis or intestinal metaplasia, (v) a family history of gastric cancer, (vi) functional dyspepsia, (vii) long-term use of nonsteroidal anti-inflammatory drugs/aspirin medication, and (viii) previous long-term use of proton pump inhibitor (PPI); H. pylori eradication is also indicated in patients with idiopathic thrombocytopenic purpura who are infected with H. pylori [4].
In the Maastricht Consensus V, H. pylori gastritis is defined as an infectious disease irrespective of symptoms and complications, and all adult patients with signs of active H. pylori infection should be diagnosed and treated. Even a test and treat strategy is considered appropriate for relatively young adult patients with uninvestigated dyspepsia in high prevalence H. pylori populations. However, an endoscopy-based strategy should be considered in patients with dyspeptic symptoms to rule out significant esophageal pathologies, particularly in low prevalence H. pylori populations. Unlike the Korea Adult Guidelines, the Maastricht Consensus V includes unexplained iron deficiency anemia despite appropriate evaluation and vitamin B12 deficiency in the indication and excludes asymptomatic individuals with family history of gastric cancer from the indication [5].
Unlike the Korean and European adult guidelines, the ESPGHAN/NASPGHAN guidelines for children and adolescents stated that the indications for endoscopic diagnosis and treatment of H. pylori infection only include gastric or duodenal ulcers and refractory iron deficiency anemia in which other etiologies have been ruled out, and noninvasive diagnostic testing for H. pylori infection may be considered when investigating the underlying causes of chronic immune thrombocytopenic purpura. It does not warrant a test and treat strategy for H. pylori infection in children. The ESPGHAN/NASPGHAN guidelines do not recommend invasive diagnostic testing for H. pylori infection in children if peptic ulcers are not clinically suspected or identified by endoscopy, in children with functional abdominal pain, in children with iron deficiency anemia as part of the initial investigation, and when investigating the causes of short stature because based on current evidence, the treatment provided to eliminate H. pylori infection is not expected to improve the symptoms and signs in children, except in cases of peptic ulcer disease. The ESPGHAN/NASPGHAN guidelines generally do not recommend an aggressive diagnostic approach to H. pylori infection in children. When H. pylori infection is confirmed by a diagnostic test, the decision to treat or not to treat should be determined after a careful discussion with parents and older patients about the advantages and disadvantages of anti-H. pylori therapy. This strategy might be helpful in the prevention of peptic ulcer and gastric cancer later in life. However, it would be accompanied with the risk of reinfection after eradication, the theoretical increased risk of allergic diseases in young children, non-relief of symptoms, treatment failure, cramps, diarrhea, and undesirable alteration of the gut microbiome after the treatment [6].
Although the H. pylori infection rate in Korea is decreasing, the seroprevalence of anti-CagA immunoglobulin (Ig) G antibodies in adolescents remains at 20%. Many Korean adolescents experience stress from academic problems and experience much more academic burnouts, such as emotional exhaustion, cynicism, and academic inefficacy, than those of developed countries [7]. Individuals with high levels of perceived everyday life stress are at increased risk of developing peptic ulcer disease [8], and stress may aggravate the severity of H. pylori infection [9]. H. pylori-infected Korean adolescents are at higher risk of progression to severe gastritis, peptic ulcer disease, atrophic gastritis, and intestinal metaplasia throughout the adolescent period than those in developed countries. Therefore, Korean adolescents with upper gastrointestinal symptoms including dyspepsia could be managed in accordance with the Korean Adult Guidelines by referring to the Maastricht Consensus V. The purpose of diagnosing the H. pylori infection in patients with gastroduodenal disorders is to basically eradicate the H. pylori and help patients recover from the disease. Frequently prescribed anti-H. pylori therapeutic agents could be administered at adult doses in adolescents aged over 10 to 12 years with body weight over 35 kg [6]. Bismuth and tetracycline, the components of the bismuth-based quadruple therapy, could be administered at adult doses in adolescents aged over 10 and 12 years, respectively. However, it remains uncertain whether sufficient anti-microbial concentrations could be maintained in the gastric mucosa in young children to eradicate H. pylori living on it when anti-H. pylori therapeutic agents are administered according to body weight-based dosing. The treatment provided to eliminate H. pylori infection is not also expected to improve symptoms in young children in developed countries. Therefore, Korean children under 10 years of age should be managed in accordance with the ESPGHAN/NASPGHAN guidelines. However, if the organic cause of abdominal pain or dyspepsia is suspected, upper gastrointestinal endoscopy should be arranged even in young children.
Diagnostic tests are usually categorized into invasive (biopsy-based) and noninvasive methods. Invasive diagnostic tests include endoscopy, culture, histology, rapid urease test (RUT), and polymerase chain reaction (PCR). Noninvasive diagnostic tests include stool antigen test (SAT), 13C-urea breath test (UBT), and serology. Each diagnostic method has its own advantages, disadvantages, and limitations (Table 1).
The ESPGHAN/NASPGHAN guidelines recommended that H. pylori infection in children should be diagnosed either by positive culture or by finding H. pylori gastritis on histopathology plus one more positive test such as RUT. If the result of histopathological examination and RUT do not match, diagnosis is determined by carrying additional noninvasive tests such as the UBT or the H. pylori SAT [6].
The Korean Adult Guidelines do not recommend a test and treat strategy for uninvestigated dyspepsia as part of the initial investigation because of the cheaper endoscopy costs than other countries [4]. As part of the initial investigation, the Maastricht Consensus V recommends a test and treat strategy for uninvestigated dyspepsia in high prevalence H. pylori populations (≥20%) and an endoscopy-based strategy in low prevalence H. pylori populations to rule out significant esophageal pathologies. However, an endoscope and treat strategy is preferred in older patients or patients with alarm symptoms or signs of gastric cancer [5]. The ESPGHAN/NASPGHAN guidelines for children do not recommend a test and treat strategy in all pediatric patients and state that the primary goal of the clinical investigation of gastrointestinal symptoms should be to determine the underlying etiology of the symptoms and not solely the presence of H. pylori infection. The guidelines do not recommend performing invasive diagnostic testing for mere detection of H. pylori infection if peptic lesions are not clinically suspected or identified by endoscopy. The diagnostic investigation for H. pylori in children is justified only in cases where the expected benefits exceed the costs and risks of testing and subsequent treatment [6].
A test and treat strategy could not be recommended in Korean children and adolescents in view of the Korean Adult Guidelines and the ESPGHAN/NASPGHAN guidelines. As part of the initial investigation, the restricted use of diagnostic approach to H. pylori detection might be applied to Korean children aged under 10 to 12 years in accordance with the ESPGHAN/NASPGHAN guidelines, and an active endoscopy-based diagnostic strategy should be applied to Korean adolescents aged over 10 to 12 years as stated in the Korea Adult Guidelines. However, biopsy-based diagnostic evaluation should be performed if the diagnostic endoscopies are carried out for any reason.
The recommended noninvasive tests useful for the test and treat strategy as part of the initial investigation in the Maastricht Consensus V are UBT, two-step monoclonal SAT, and locally validated serological tests [5].
During the initial diagnostic endoscopic examination, the Maastricht Consensus V recommends obtaining two biopsies from the antrum (greater and lesser curvature, 3 cm proximal to the pyloric region), two biopsies from the middle of the corpus, and additional biopsy from the incisura (5) for histology; one biopsy from the corpus and one from the antrum (2) for RUT; and one gastric biopsy specimen (1) for culture followed by MIC determination or molecular test to verify the clarithromycin susceptibility if clarithromycin resistance is >15% [5]. The ESPGHAN/NASPGHAN guidelines recommended obtaining two biopsies from the antrum and two biopsies from the corpus (4) for histological evaluation, at least one biopsy from the antrum and one from the corpus (2) for culture (if available), and at least one biopsy (1) for any additional diagnostic tests from the antrum for RUT or molecular-based assays [6].
Endoscopy-based diagnostic methods such as culture, histology, and RUT and noninvasive methods such as SAT and UBT should be performed to avoid false-negative test results at least 2 weeks after stopping PPI and 4 weeks after stopping antibiotics.
Conventional endoscopy in adults is usually performed to diagnose H. pylori-related diseases, such as peptic ulcer diseases, atrophic gastritis, MALT lymphoma, and gastric cancer. Although several attempts including a careful close-up observation of the gastric mucosal findings, such as redness, mucosal swelling, or nodular change, with standard endoscopy have been made for real-time diagnosis of H. pylori infection during endoscopic examination, those attempts may be time consuming and do not provide better results than other biopsy-based tests. In addition to conventional endoscopy, chromoendoscopy with phenol red, magnifying endoscopy with or without indigo carmine staining, confocal laser endomicroscopy, magnifying endoscopy with narrow band imaging, and magnifying endoscopy with I-scan have been used to detect H. pylori infection in adults. However, highly skilled operators are required to operate these advanced technologies, and a careful examination using magnifying with or without image-enhanced technique is also time consuming and may make a patient more uncomfortable compare with other biopsy-based tests. Those factors usually limit the clinical use of magnifying endoscopy to detect H. pylori infection in the clinical practice [10]. In adults, it is recommended to collect biopsies of even normal-appearing gastric mucosa for H. pylori detection during endoscopy in patients with dyspeptic symptoms [11].
The recent advancement in medical technology had made endoscopic procedures feasible not only in young children but also in newborns. The endoscopic findings of an H. pylori-infected gastric mucosa in children are distinct from those seen in adults. If there are nodular changes in the antrum or erosions and ulcers in the stomach or duodenum, H. pylori infection should be suspected in children. A distinct nodular gastritis, rarely identified during adult endoscopic examinations, is a common endoscopic manifestation and may be a pathognomonic finding of H. pylori infection in children [12]. In a conventional or magnifying endoscopy, the absence of regular arrangement of collecting venules on the corpus mucosa in children could be associated with H. pylori infection as in adults [13].
Culturing of H. pylori from gastric biopsy specimen is the gold standard and the most specific method but the sensitivity of culture shows significant variations. Detection of H. pylori through cultural isolation is expensive, relatively difficult to perform, and time-consuming, and represents a procedure that requires equipment and microbiological expertise, and it is significantly influenced by the conditions of transport of the biopsy specimen from the endoscopy unit to the microbiology laboratory.
To obtain proper biopsy specimen for the high-yield H. pylori isolation, the endoscopic instrument including the biopsy channel must be vigorously washed with anti-septic solutions with normal saline and biopsy should be performed before the biopsy forceps are contaminated with formalin. The obtained biopsy specimens for culture should be placed quickly into a container with a few drops of saline and transferred to the microbiology laboratory by experienced microbiologists.
The Maastricht Consensus V recommended that H. pylori culture and antibiotic susceptibility testing should be performed with one gastric biopsy specimen if primary resistance to clarithromycin is higher than 15% in a given geographical area, after the first failure to tailor the treatment, except if a bismuth-based quadruple therapy is considered, or after failure of second-line treatments as a treatment guide [5]. The ESPGHAN/NASPGHAN guidelines recommend obtaining one biopsy from the antrum and one from the corpus for culture, obtaining information about the antimicrobial sensitivity of the infecting H. pylori strain(s), and ensuring that eradication therapy should be tailored accordingly as an initial approach for diagnosing H. pylori infection in children [6]. The guidelines for children recommend obtaining biopsy specimens from two places because several strains with different antibiotic sensitivity profiles can exist within the same child [6]. However, compared with the Maastricht Consensus V and the ESPGHAN/NASPGHAN guidelines, the Korean Adult Guidelines do not recommend H. pylori culture for antimicrobial sensitivity as the initial diagnostic approach. It was not also recommended for Korean children and adolescents because the manpower and laboratory facilities with expertise in H. pylori culture are only available in few research laboratories in Korea. However, considering the increasing failure rate of standard therapies due to increased antibiotic resistance in Korea, bacterial culture will serve as an important method to prevent treatment failures. Therefore, several regional microbiology laboratories with a good quality assurance and quality control program shall be established so that the transfer of the biopsy specimens for H. pylori culture and MIC determination at least after failure of second-line treatment could be possible.
Biopsy specimens could be transferred to a distant microbiology laboratory for successful H. pylori isolation if kept in a conventional transport medium, such as Portagerm pylori, Stuart's transport medium, serum-free transport medium with cyanobacterial extract, brain heart infusion broth plus vancomycin or amphotericin B, and nalidixic acid for up to 24 hours at 4℃, and a newly developed semisolid GESA transport medium for up to 10 days at 4℃ [14].
Standard hematoxylin and eosin staining of the gastric tissue is accepted as one of the good sensitive methods to detect H. pylori infection. It provides additional information about the presence and severity of inflammation, atrophy, and intestinal metaplasia based on the updated Sydney System in which gastric biopsy specimens are collected from the following five sites: the lesser curvature of the corpus approximately 4 cm proximal to the angulus (i), from the lesser (ii) or greater curvature of the antrum (iii), both within 2 to 3 cm of the pylorus, from the middle portion of the greater curvature of the corpus, approximately 8 cm from the cardia (iv), and from the incisura angularis (v). The ESPGHAN/NASPGHAN guidelines for children, in whom intestinal metaplasia is very rare, recommended obtaining two biopsies from the antrum and two biopsies from the corpus for histological evaluation. Obtaining at least two biopsy specimens from the antrum and the corpus, respectively, increases the sensitivity to detect H. pylori. The corpus biopsy is important for the diagnosis of H. pylori in patients with atrophic gastritis. The sensitivity and specificity to detect H. pylori infection can be improved by using special stains such as modified Giemsa, Warthin-Starry silver, Genta, and immunohistochemical stains. It is known that the accuracy is affected by preceding antibiotics, bismuth, and PPIs and that the histological methods need skilled personnel for specimen processing and intrinsically have an inter-observer variability in the diagnosis [15]. In a study comparing Giemsa and immunohistochemistry (IHC) to diagnose H. pylori infection, Giemsa showed significantly lower sensitivity (83.3%) than IHC (98.8%). Moreover, the sensitivity of Giemsa markedly dropped to 33.6% in patients without active inflammation. The sensitivity of Giemsa strongly depends on H. pylori density and, accordingly, on the presence of active inflammation [16]. IHC has been proved to increase the sensitivity for detection of H. pylori infection, and this method has a lower inter-observer variation when compared to histochemical stains. The use of IHC could be recommendable in patients with chronic active or inactive gastritis, atrophic gastritis, intestinal metaplasia, or in follow-up biopsies after the eradication of H. pylori, when no organisms are identified on histochemical staining [5].
H. pylori is usually observed in the gastric mucosa. However, the mucous layer, together with the bacteria, is lost during conventional tissue processing in which formalin is used for fixation. When the number of H. pylori in the gastric mucosa is limited as in pediatric patients, the bacteria may not be detected by conventional histologic methods. The preservation of mucous layer using Carnoy's solution as a fixative and the following immunohistochemical detection of H. pylori might aid in further increasing the diagnostic yield in pediatric patients [17].
Peptide nucleic acid fluorescent in situ hybridization (PNA-FISH) is highly sensitive and specific when it comes to detecting H. pylori infection and can identify coccoid forms of H. pylori. Recently, PNA-FISH is increasingly used to detect H. pylori clarithromycin resistance in gastric biopsy specimens [10].
In routine clinical practice, the RUT is the most useful invasive test for the diagnosis of H. pylori infection because it is a simple and inexpensive method that requires no special technique to perform and read the result. The biopsy specimen is placed into a solution or gel-containing urea and a pH indicator. If H. pylori is present, the urea is broken down into CO2 and ammonia, which increases the pH of the solution or gel and causes a subsequent color change in the pH indicator. The RUT produces a result in a range of minutes up to 24 hours, depending on the number of bacteria in the biopsy specimen. A positive RUT may require approximately 103 to 105 H. pylori in the biopsy sample to change the color depending on the experimental conditions [1819].
In the Maastricht Consensus V, the RUT is recommended as a first-line diagnostic test in the endoscopy-based strategy when there is no contraindication for biopsy. In cases of positive tests, an immediate treatment against H. pylori infection is guaranteed. One biopsy should be taken from the corpus and one from the antrum. RUT is not recommended for the evaluation of H. pylori eradication after treatment [5]. In the ESPGHAN/NASPGHAN guidelines, at least one biopsy for RUT or molecular-based assay from the antrum is recommended [6].
Multiple RUTs were performed in 50 pediatric patients, consisting of 25 initial RUT-positive and 25 RUT-negative patients, with more than 5 gastric antral biopsy specimens collected from October 1991 to April 1992 and stored frozen in the Biobank to explore the nature of patchy distribution of H. pylori infection and to verify whether these are more accentuated in children. Of 50 patients, 30 showed consistent results on all RUTs whereas 20 showed inconsistent results. The result of RUT was determined positive if at least one out of six or more RUTs yielded positive results, and 32 out of 50 patients were considered as RUT positive. Some RUTs revealed negative results in 20 out of 32 RUT-positive cases. Therefore, RUTs using one biopsy specimen might show up to 62.5% false-negative results (Fig. 1) (unpublished observation).
The RUT using one and three biopsy specimens from each of the 255 children were compared to check the correlation between the number of gastric biopsy specimens and the positivity rate. The positivity rate of the RUTs with three biopsy specimens was higher than with RUTs with one biopsy specimen. The difference between one and three biopsy specimens was higher in the children aged 0 to 9 years. The RUT might be a more accurate diagnostic modality when it is performed with three or more biopsy specimens especially in children (Fig. 2) [20]. The differences in the results of RUTs according to age and sampling site were also evaluated. The positive color change of the RUTs in adults primarily occurred within 1 hour both in the antrum and in the corpus. The proportions of positive RUT reaction within 1 hour were similar between the antrum and the corpus regardless of age. The positive color change of the RUTs in children primarily occurred in the corpus within 6 to 24 hours. The discrepancy in the positivity rates between the antrum and the corpus in children was mainly due to the difference in the proportion of positive reactions in the RUTs within 6 to 24 hours (Fig. 3) [21]. The higher positivity rate of corpus RUTs than of antrum RUTs in children, although positive color change did not occur within 6 hours, might be related to the increased H. pylori urease activity at the corpus where parietal cells secrete acid into the mucous layer. Therefore, the RUT may be more accurate when three or more specimens from both the antrum and corpus are used in children.
However, there is a high possibility of false-negative results with RUT due to decreased urease activity, which could be caused by a recent intake of antibiotics, bismuth compounds, or PPIs. Moreover, a false-negative urease test can be observed in patients with achlorhydria and bleeding. The risk of a false-positive result in RUTs may increase with increasing incubation time [15].
PCR provides excellent sensitivity and specificity compared with other conventional tests and can accurately detect the presence of H. pylori even in patients with bleeding. PCR also enables clinicians to make fast and more accurate decisions on patient's treatment. The target genes such as ureA, glmM, ureC, 16S rRNA, 23S rRNA, hsp60, and vacA are frequently used in the diagnosis of H. pylori infection using PCR [10]. The conventional PCR might be helpful in the diagnosis of H. pylori infection even when few organisms exist in the gastric biopsy specimen and even if other biopsy-based tests are expected to show false-negative results. However, it has a high risk of false-negative and false-positive results, unless standard precautions are observed during specimen collection and PCR procedures. The presence of cross-reacting nucleotide sequences and the high genetic variability of the bacterial strains as well as the DNA segments of dead bacterium in the gastric mucosa of patients after treatment or if dead or live H. pylori strains remain in the biopsy channel are among the limitations of PCR-based diagnosis [22].
Detection of virulence factors such as cagA, vacA, or dupA by PCR helps to evaluate the genetic variation within virulence factors of H. pylori and provides more information to understand the clinical discrepancies among H. pylori-infected patients. It is also useful in epidemiological studies [10].
Furthermore, it can be used to identify specific mutations associated with resistance to antimicrobial agents such as clarithromycin (23S rRNA), quinolones (gyrA), tetracycline (16S rRNA), rifabutin (rpoB) and amoxicillin (pbp-1a) [10]. This antibiotic resistance profile may provide an important information for the clinicians to determine the anti-H. pylori therapeutic strategy.
The recent progress in the PCR procedures and the invention of real-time PCR made the molecular method useful in the quantitative detection of H. pylori in biopsy specimens following anti-H. pylori therapy as well as in stool, dental plaque, saliva, and environmental samples with high sensitivity and high specificity [10]. Many research papers using PCR have been published, and many of them used stools instead of biopsies to diagnose H. pylori infection.
SAT can detect bacterial antigens of H. pylori and therefore can be used in the initial and post-treatment diagnosis of H. pylori infection. There are two types of SATs used for H. pylori detection. One is an enzyme immunoassay (EIA), the so-called two-step SAT, and the other is an immunochromatography assay (ICA), the so-called one-step SAT. Both types use either polyclonal or monoclonal antibodies. In general, monoclonal antibody-based tests are more accurate than polyclonal antibody-based tests, and EIA-based tests provide more reliable results than ICA-based tests. The rapid monoclonal ICA-based SAT has a high specificity but has a low sensitivity [10]. The ICA-based tests may have problems in interpreting weakly positive results [23]. The sensitivity and specificity of the laboratory-based monoclonal EIA are comparable to those of UBT. Monoclonal SAT is the recommended noninvasive test in the context of a test and treats strategy in adults [5].
In addition to assessment of eradication therapy, monoclonal SAT is a convenient and useful test for the initial diagnosis of H. pylori infection in pediatric patients. The use of SAT in the diagnosis of H. pylori has theoretically no age restriction and needs no starvation before the diagnosis. Stool samples could be stored for 24 hours at room temperature or 72 hours at 4℃ before SAT.
The accuracy of SATs is decreased when the stool samples are unformed or watery, because H. pylori-specific antigens in the stool samples are diluted [10]. False-negative results are also expected in patients with conditions that can decrease bacterial loads in the gastric mucosa, such as use of PPIs, use of antibiotics, bleeding peptic ulcer, atrophic gastritis with or without intestinal metaplasia, gastric cancer, MALT lymphoma, and partial gastrectomy [24].
The UBT is based on the ability of H. pylori, if present in the gastric environment, to break down orally absorbed 13C-urea into 13CO2 and ammonia. 13CO2 is absorbed in the blood, exhaled in the breath, and measured in the exhaled air. The test should be conducted under fasting conditions to optimize the contact of the test solution with the gastric mucosa.
UBT is the most investigated and best recommended noninvasive test to diagnose H. pylori infection in the context of a test and treat strategy [5]. It is useful in both pre- and post-treatment diagnosis and used to detect reinfection 1 and 2 years after H. pylori eradication. The relatively good sensitivity of the UBT, especially after eradication therapy, may be explained by the fact that the UBT is more likely to produce positive results than biopsy-based tests in cases of moderate colonization or patchy distribution of H. pylori [15]. While not a true quantitative test, 13C-UBT can be used to evaluate semi-quantitatively the load of H. pylori in the stomach. The UBT is an appropriate method with many advantages, such as simplicity and safety, to detect H. pylori infection in pediatric patients, although the accuracy of UBT in pediatric patients is not as good as that in adult patients [10].
Just like in SATs, false-negative results might be observed in patients with the following clinical conditions that can decrease bacterial loads in the stomach mucosa, such as use of PPIs, use of antibiotics, bleeding peptic ulcer, atrophic gastritis with or without intestinal metaplasia, gastric cancer, MALT lymphoma, and partial gastrectomy [24].
False-positive results were observed in case of adult patients with acid-free stomach, caused by atrophic gastritis or a long-term use of PPIs, where urease-positive bacterial species such as Proteus mirabilis, Citrobacter freundii, Klebsiella pneumoniae, Staphylococcus aureus, and Enterobacter cloacae were cultured. These bacterial species with urease activity probably originated from the patient's oral cavity [24]. However, in children aged less than 6 years, the clinical application of UBT also has a limited value because of the relatively low specificity and high rate of false-positive results compared with adults and older children. The smaller stomach of the children, especially those aged less than 6 years, has a lower distribution volume of ingested 13C-urea solution and the endogenous 12CO2 production rate is lower in young children than in older children or adults. Therefore, the ingestion of an identical amount of 13C-urea regardless of age may increase the isotopic ratio of 13CO2/12CO2 in younger children compared with old children or adults [12]. To decrease the false-positive rate in children aged less than 6 years, increasing the cutoff value from 2.4‰-4.0‰ to 7.0‰ was suggested in this age group. Another explanation for high false-positive results in young children is the presence of urease-producing microorganisms in the oral cavity, as young children are unwilling to swallow 13C-urea during the test procedure [25].
Numerous serological tests based on the detection of anti-H. pylori IgG antibody are widely available for H. pylori diagnosis, and enzyme-linked immunosorbent assay (ELISA) test is the most common and accurate technique among them. The serological tests have been used for epidemiological studies, screening, initial diagnosis, or confirmation of another test because of their low cost, simplicity, and acceptability to patients. Serology usually does not reliably distinguish between active and past infection. However, a quantitative ELISA test detecting the difference between anti-H. pylori IgG titers of paired sera from the acute and convalescent (6 to 12 months) phase can confirm eradication of the infection [5].
The differences in sensitivity and specificity of ELISA, using the same commercial kit, in different adult populations have been reported. The commercially available serologic assays have not been proven to have the necessary sensitivity or specificity to screen young pediatric patients. The mean serologic antibody titers among infected pediatric patients are lower than the mean titers in infected adult patients [26]. For these reasons, it has been suggested that the test must be validated in local adult and pediatric populations [27]. An ELISA using whole-cell extracts was validated in 50 children with biopsy-confirmed infection. The whole-cell extract ELISA after resetting the cutoff value in the study population showed an improved sensitivity and specificity [28].
The serology can be used in the diagnosis of H. pylori infection even when there are significant gastric mucosal changes that may lead to a low bacterial load in the stomach and to decreased sensitivity of other diagnostic methods. These significant gastric mucosal changes include GI bleeding, atrophic gastritis, gastric MALT lymphoma, and gastric carcinoma [5].
The Maastricht Consensus V stated that the H. pylori serology kits should ideally be developed using local H. pylori strains, local titers should be established, and all H. pylori serology kits should be locally validated because regional differences in prevalence of infection, infection load, and strain distribution are likely to exist [5]. In the clinical setting, however, the ESPGHAN/NASPGHAN guidelines for children do not recommend the use of serum, whole blood, urine, and saliva in antibody-based tests (IgG, IgA) for H. pylori [6]. Office-based whole blood tests and antibody detection in urine or saliva are considered less accurate and should not be recommended to diagnose H. pylori infection in adults [15].
CagA is an important virulence factor in H. pylori, and most of H. pylori isolates in Korea possess cagA gene [29]. Therefore, antibody tests for detection of antibody response against regional CagA antigens with high sensitivities and specificities have been used in the serological diagnosis of H. pylori infection [3031].
In Korea, four commercially available ELISA kits were compared with immunoblotting in detection of anti-H. pylori IgG antibodies. Gap (Bio-Rad, Richmond, CA, USA), HMCAP (Enteric Products, Westbury, NY, USA), Pyloriset EIA-G (Orion, Espoo, Finland), and Genedia H. pylori ELISA (Green Cross Medical Science Corp., Eumseong, Korea) were evaluated using a total of 679 sera collected from Koreans from 1998 to 1999 and interpreted according to the manufacturer's instructions. IgG immunoblotting to detect anti-CagA antibody was also performed using a sonicated crude H. pylori antigen and 1:5 serum dilution. Immunoblotting showed the highest seropositive rate in all age groups. The gaps of seropositivity between ELISA tests and immunoblotting were wider among children aged 1 to 5 years. Among four ELISA kits, Genedia IgG kit, made of H. pylori strains isolated in Korea, has the highest seropositivity compared with the three other kits imported from abroad. The results emphasize the need for standardization when the commercial ELISA tests are used in different countries or in young age groups (Fig. 4) [32].
Detection of patchily distributed, small number of H. pylori would be expected to be unsatisfactory in assessing efficacy using biopsy specimens after anti-H. pylori therapy. Post-treatment diagnosis for H. pylori eradication should also be carried out at least 4 weeks after completion of antibiotic therapy and after PPI therapy have been withheld for 2 weeks to decrease the false negative results. In the Maastricht Consensus V, UBT is the best option for confirmation of H. pylori eradication, and monoclonal EIA-based SAT is an alternative method. In addition, IHC might be suitable for follow-up biopsies after eradication treatment for H. pylori. However, RUT is not recommended to evaluate H. pylori eradication after treatment [5]. In the ESPGHAN/NASPGHAN guidelines for children, the outcome of anti-H. pylori therapy should be assessed using a 13C-UBT or a monoclonal EIA-based SAT [6]. Real-time PCR using gastric biopsy specimens would be an alternative and useful method to detect a very small number of H. pylori left.
In Korea Adult Guidelines, the outcome of anti-H. pylori therapy should be assessed at least 4 weeks after completion therapy using either one of the noninvasive methods (UBT or SAT) or invasive methods (RUT or histology). The recommendations of Korean adult guidelines include an invasive test for the post-treatment evaluation because the endoscopic examinations are more common and cost-effective compared with the methods used in other countries and because histologic changes as well as endoscopic findings are important for Korean adults. Each biopsy sample should be taken from the antrum and body for RUT or histology. Acid suppressants should be stopped at least 2 weeks before UBT [4].
In Japan, the national health insurance system covers either one of three invasive methods (culture, RUT, and histology) or two of three noninvasive methods (SAT, UBT, and serum anti-H. pylori antibody titer) for post-treatment evaluation. A combination of two noninvasive methods is recommended more than one invasive test, and a reduction in serum IgG antibody titer of more than 50% from its initial level at least 6 months after completion treatment is the most reliable method to confirm that eradication treatment was successful [33].
Figures and Tables
 | Fig. 1A comparison of the results of multiple rapid urease tests (RUTs) between 25 initial RUT-positive (A) and 25 RUT-negative (B) pediatric patients from whom five or more gastric antral biopsy specimens were also available for 13C-urea breath tests revealed that RUTs using one biopsy specimen show a maximum of 62.5% false-negative results. |
 | Fig. 2The difference in the positivity rate of rapid urease tests (RUTs) between one and three biopsy specimens obtained from the gastric antrum in the same pediatric patient according to the age groups. The positivity rate of RUTs increased with age irrespective of the number of gastric biopsy specimens. The positivity rate of RUTs with three biopsy specimens was higher than RUTs with one biopsy specimen in patients aged below 10 years of age [20].GU1: RUT using one biopsy specimen, GU3: RUT using three biopsy specimens.
|
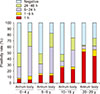 | Fig. 3The positivity rate and time to positivity of rapid urease tests (RUTs) in the antrum and body according to age. The positivity rate of RUTs in the antrum was higher in the age group 20 to 29 years compared with that in other three age groups, and the positivity rate of RUTs in the body decreased with increasing age (p<0.0001). The most frequent time to positivity occurred within 1 hour in the age group 20 to 29 years and within 6 to 24 hours in children (p<0.0001). The proportions of positive reactions within 1 hour were similar for the antrum and body in all groups [21]. |
 | Fig. 4Seropositivity rates of the four commercial enzyme-linked immunosorbent assay kits and immunoblotting according to age. The seropositivity rates increased with age. The seropositivity rates of immunoblotting were higher than those of the ELISA kits, and the discrepancy in the seropositivity rates of anti-Helicobacter pylori immunoglobulin G (IgG) antibody was highest in the 0-to-6-month-old infants [32]. |
Table 1
Diagnostic Tests for the Detection of Helicobacter pylori Infection

ESPGHAN: European Society of Paediatric Gastroenterology, Hepatology and Nutrition, NASPGHAN: North American Society of Paediatric Gastroenterology, Hepatology and Nutrition, IHC: immunohistochemistry, PPI: proton pump inhibitor, MALT: mucosa-associated lymphoid tissue, Ig: immunoglobulin, GI: gastrointesinal, ELISA: enzyme-linked immunosorbent assay.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2010-0011777).
References
1. Rhee KH, Youn HS, Baik SC, Lee WK, Cho MJ, Choi HJ, et al. Prevalence of Helicobacter pylori infection in Korea. J Korean Soc Microbiol. 1990; 25:475–490.
2. Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. J Infect Dis. 1993; 168:219–221.

3. Kuipers EJ, Peña AS, van Kamp G, Uyterlinde AM, Pals G, Pels NF, et al. Seroconversion for Helicobacter pylori. Lancet. 1993; 342:328–331.

4. Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, et al. Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. Korean J Gastroenterol. 2013; 62:3–26.

5. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017; 66:6–30.

6. Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017; 64:991–1003.

7. Lee JY, Puig A, Kim YB, Shin HJ, Lee JH, Lee SM. Academic burnout profiles in Korean adolescents. Stress Health. 2010; 26:404–416.

8. Deding U, Ejlskov L, Grabas MP, Nielsen BJ, Torp-Pedersen C, Bøggild H. Perceived stress as a risk factor for peptic ulcers: a register-based cohort study. BMC Gastroenterol. 2016; 16:140.

9. Jia K, An L, Wang F, Shi L, Ran X, Wang X, et al. Aggravation of Helicobacter pylori stomach infections in stressed military recruits. J Int Med Res. 2016; 44:367–376.

10. Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015; 21:11221–11235.

11. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017; 112:212–239.

12. Yang HR. Updates on the diagnosis of Helicobacter pylori infection in children: what are the differences between adults and children? Pediatr Gastroenterol Hepatol Nutr. 2016; 19:96–103.

13. Hidaka N, Nakayama Y, Horiuchi A, Kato S, Sano K. Endoscopic identification of Helicobacter pylori gastritis in children. Dig Endosc. 2010; 22:90–94.
14. Cellini L, Di Campli E, Di Bartolomeo S, Bessa LJ, Baffoni M, Di Giulio M. New transport medium for cultural recovery of Helicobacter pylori. J Clin Microbiol. 2014; 52:4325–4329.

15. Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol. 2014; 20:1438–1449.

16. Kocsmár É, Szirtes I, Kramer Z, Szijártó A, Bene L, Buzás GM, et al. Sensitivity of Helicobacter pylori detection by Giemsa staining is poor in comparison with immunohistochemistry and fluorescent in situ hybridization and strongly depends on inflammatory activity. Helicobacter. 2017; 22:e12387.
17. Kim YK, Lee JS, Kim HW, Lee JH, Youn HS, Ko GH. Detection of Helicobacter pylori in the gastric mucous layer in pediatric patients. Korean J Pathol. 2002; 36:292–295.
18. Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am. 2000; 29:871–878.
19. Uotani T, Graham DY. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 2015; 3:9.
20. Seo JH, Park JS, Yeom JS, Lim JY, Park CH, Woo HO, et al. Correlation between positive rate and number of biopsy samples on urease test in childhood Helicobacter pylori infection. J Korean Med Sci. 2014; 29:106–109.

21. Seo JH, Youn HS, Park JJ, Yeom JS, Park JS, Jun JS, et al. Influencing factors to results of the urease test: age, sampling site, histopathologic findings, and density of Helicobacter pylori. Pediatr Gastroenterol Hepatol Nutr. 2013; 16:34–40.

22. Cho YK, Jung YS, Lim JY, Kim YO, Choi MB, Park CH, et al. Validity of polymerase chain reaction in the diagnosis of Helicobacter pylori infection using gastroscopic bilpsy specimens in children. Korean J Gastroenterol. 1998; 31:16–22.
23. Moon DI, Shin EH, Oh HG, Oh JS, Hong S, Chung Y, et al. Usefulness of a Helicobacter pylori stool antigen test for diagnosing H. pylori infected C57BL/6 mice. Lab Anim Res. 2013; 29:27–32.

24. Syrjänen K. False positive and false negative results in diagnosis of Helicobacter pylori infection can be avoided by a panel of serum biomarkers (GastroPanel®). M J Gast. 2017; 2:007.
25. Yang HR, Seo JK. Diagnostic accuracy of the C-urea breath test in children: adjustment of the cut-off value according to age. J Gastroenterol Hepatol. 2005; 20:264–269.

26. Czinn SJ. Serodiagnosis of Helicobacter pylori in pediatric patients. J Pediatr Gastroenterol Nutr. 1999; 28:132–134.

27. de Oliveira AM, Rocha GA, Queiroz DM, Mendes EN, de Carvalho AS, Ferrari TC, et al. Evaluation of enzyme-linked immunosorbent assay for the diagnosis of Helicobacter pylori infection in children from different age groups with and without duodenal ulcer. J Pediatr Gastroenterol Nutr. 1999; 28:157–161.

28. Camorlinga-Ponce M, Torres J, Perez-Perez G, Leal-Herrera Y, Gonzalez-Ortiz B, Madrazo de, et al. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am J Gastroenterol. 1998; 93:1264–1270.

29. Youn HS, Baik SC, Cho YK, Woo HO, Ahn YO, Kim K, et al. Comparison of Helicobacter pylori infection between Fukuoka, Japan and Chinju, Korea. Helicobacter. 1998; 3:9–14.

30. Seo JH, Lim CW, Park JS, Yeom JS, Lim JY, Jun JS, et al. Correlations between the CagA antigen and serum levels of anti-Helicobacter pylori IgG and IgA in children. J Korean Med Sci. 2016; 31:417–422.

31. Youn HS, Baik SC, Lee WK, Cho MJ, Ryou HH, Choi HJ, et al. Serodiagnosis of Helicobacter pylori infection. J Korean Soc Microbiol. 1990; 25:463–474.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download