Abstract
Purpose
Adverse drug reactions (ADRs) are a recurring problem among pediatric patients, and the incidence is increasing. How-ever, there have been only a few studies on the clinical presentation of pediatric ADRs in Korea. This study investigated the clinical presentation of ADRs and the causative drugs in pediatric patients from a single university hospital.
Methods
We retrospectively collected the data on pediatric ADRs as reported to the Regional Pharmacovigilance Center in Dong-A University Hospital between March 2013 and July 2016. We analyzed clinical presentations associated with the events. To determi-nate causality, we evaluated each ADR according to the Naranjo probability scale, the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria and the Korean ADR algorithm.
Results
A total of 365 ADR cases were reported. Sixty-eight patients (26.5%) responded to 2 or more drugs. Antibiotics (43.3%) were the most common causative drugs, of whom the third generation cephalosphorins caused most ADRs. The most common clinical presentations were gastrointestinal manifestations (36.6%). A total of 312 ADRs were reported in 257 patients based on both the Naranjo probability scale and the Korean ADR algorithm. In addition, 323 ADRs were reported in 257 patients based on the WHO-UMC criteria.
Go to : 
REFERENCES
1. International drug monitoring: the role of national centres. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1972; 498:1–25.
2. Korea Institute of Drug Safety & Risk Management. Pharmacovigilance terminology [Internet]. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;[cited 2018 Feb 13]. Available from. https://www.drugsafe.or.kr/iwt/ds/ko/information/EgovDrugWatchTerm.do.
3. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a metaanalysis of prospective studies. JAMA. 1998; 279:1200–5.

4. Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998-2005. Arch Intern Med. 2007; 167:1752–9.

5. Chung EH, Choi YJ, Suh DI, Woo SI, Cho HJ, Chung SY. Adverse drug reactions in Korean children: an analysis of KAERS database on 2012-2013 [abstract]. Program and Abstract, the 65th Annual Fall Meeting of the Korean Pediatric Society. 2015 Oct 22-23; Seoul, Korea.Seoul (Korea): The Korean Pediatric Society;2015. p. 65.
6. Roughead E, Pratt N, Peck R, Gilbert A. Improving medication safety: influence of a patient-specific prescriber feedback program on rate of medication reviews performed by Australian general medical practitioners. Pharmacoepidemiol Drug Saf. 2007; 16:797–803.

7. Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999; 88:965–8.

8. Choonara I, Conroy S. Unlicensed and off-label drug use in children: implications for safety. Drug Saf. 2002; 25:1–5.

9. Kim MH, Jung HY, Sohn MK, Kim SE, Lee YW, Park JW, et al. Clinical features of adverse drug reactions in a tertiary care hospital in Korea. Korean J Asthma Allergy Clin Immunol. 2008; 28:35–9.
10. Kim MG, Kang HR, Kim JH, Ju YS, Park SH, Hwang YI, et al. Analysis of adverse drug reactions collected by an electronic reporting system in a single hospital. Korean J Med. 2009; 77:601–9.
11. Kim JE, Kyun JO, Jin SM, Lee YH, Kim JH, Lee HY, et al. Adverse drug reactions in elderly inpatients: comparison with younger patients. Korean J Asthma Allergy Clin Immunol. 2010; 30:216–21.
12. Choi JH, Shin YS, Suh CH, Nahm DH, Park HS. The frequency of adverse drug reactions in a tertiary care hostpital in Korea. Korean J Med. 2004; 67:290–6.
13. Park GM, Park JH, Jung JW, Han HW, Kim JY, Lee E, et al. Pediatric adverse drug reactions collected by an electronic reporting system in a single tertiary university hospital. Allergy Asthma Respir Dis. 2016; 4:354–9.

14. Adverse Drug Reaction Assessment Report. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;2013. p. 61.
15. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–45.

16. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–9.

17. Hong KS, Park BJ, Sin SG, Yang JS, Lee SM, Kim YN, et al. Development of a Korean algorithm for causality assessment of adverse drug reactions. Korean J Clin Pharmacol Ther. 2002; 10:129–42.

18. Rew SY, Koh YI, Shin HY, Park SH, Ryu SH, Kim HN, et al. Reporting and clinical features of adverse drug reactions from a single university hospital. Korean J Asthma Allergy Clin Immunol. 2011; 31:184–91.
19. Kim HM, Seong JM, Yang BR, Jin XM, Choi NK, Lee J, et al. Analysis of adverse events reporting patterns and signal detection for pediatric patients in the Korean spontaneous reporting data. Korean Soc Pharmacoepidemiol Risk Manage. 2012; 5:40–5.
20. Ferrajolo C, Capuano A, Trifirò G, Moretti U, Rossi F, Santuccio C. Pediatric drug safety surveillance in Italian pharmacovigilance network: an overview of adverse drug reactions in the years 2001-2012. Expert Opin Drug Saf. 2014; 13(Suppl 1):S9–20.
22. Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000-2009. Br J Clin Pharmacol. 2012; 73:437–46.

23. Ahn BS, Chae YM, Park MS, Shim JY, Lim SH. Development of a new adverse drug reaction monitoring system in general hospital and evaluation of its outcome. J Korean Soc Med Inform. 1999; 5:149–58.

24. Rhew KY, Lee SH. Analysis of the Korea Food and Drug Administration adverse drug reaction reports. Korean J Clin Pharm. 2011; 21:138–44.
25. Sacilotto K, Bagheri H, Lapeyre-Mestre M, Montastruc JL, Montastruc P. Adverse drug effect notifications by nurses and comparison with cases reported by physicians. Therapie. 1995; 50:455–8.
26. Son MK, Lee YW, Jung HY, Yi SW, Lee KH, Kim SU, et al. Comparison of the Naranjo and WHO-Uppsala Monitoring Centre criteria for causality assessment of adverse drug reactions. Korean J Med. 2008; 74:181–7.
Go to : 
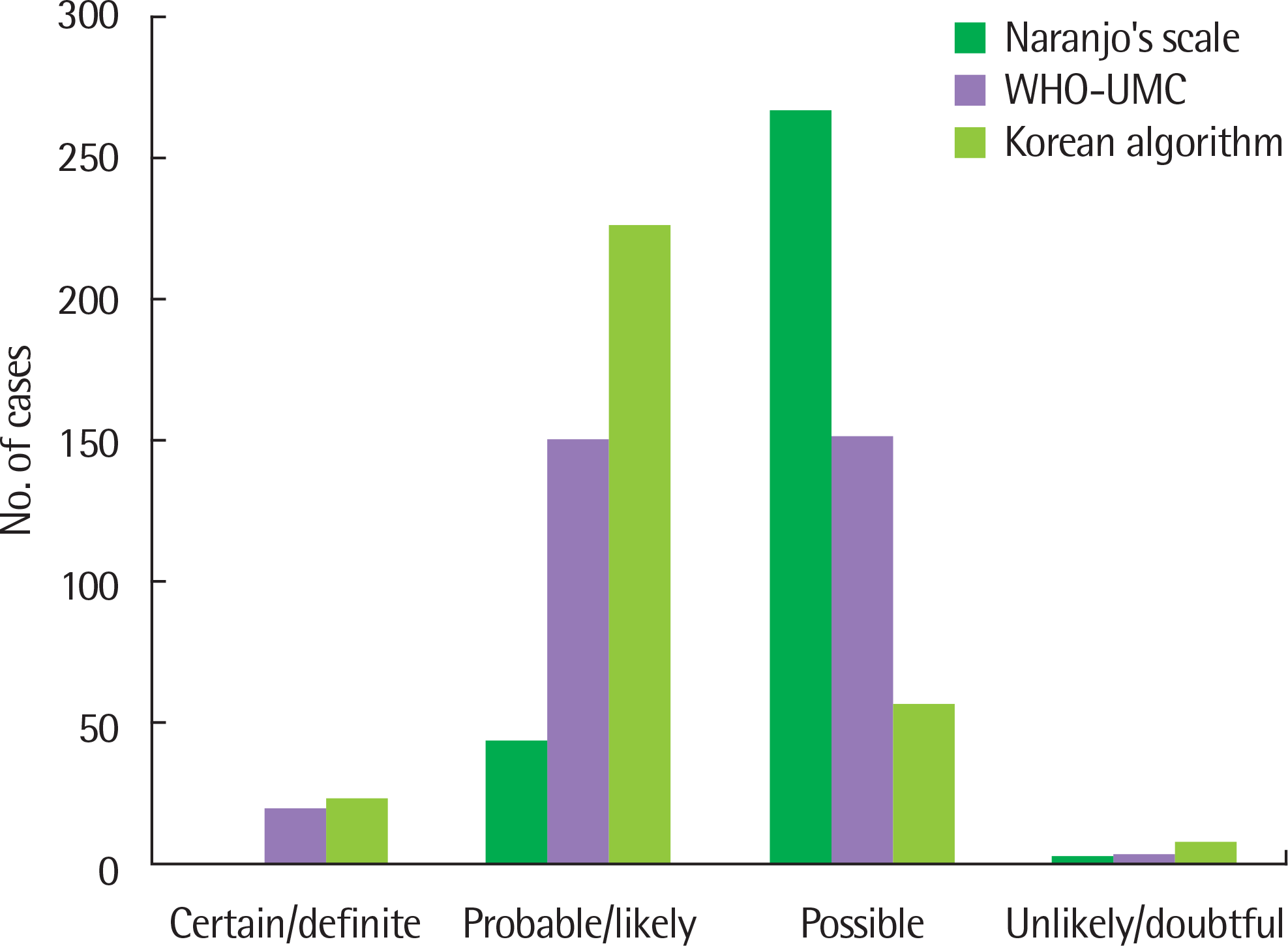 | Fig. 1.Causality assessment of adverse drug reactions by Naranjo's scale, WHO-UMC, and Korean algorithm, respectively. WHO-UMC, World Health Organization-Uppsla Monitoring Centre. |
Table 1.
Demographic data of the study subjects
Table 2.
Frequency of drug to cause the adverse reactions by age distribution
Table 3.
Frequency of drug to cause the adverse reactions
Table 4.
Clinical manifestation and severity of adverse drug reaction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download