Abstract
Purpose
Mycoplasma pneumoniae (MP) is a common cause of community-acquired pneumonia (CAP) in children. MP serum IgM and polymerase chain reaction (PCR) are the methods that enable early diagnosis in patients with MP pneumonia. The objective of this study was to investigate the clinical value of serum MP-specific IgM antibodies in PCR-positive MP pneumonia for the early diagnosis of MP pneumonia in children with CAP.
Methods
Out of 129 patients with lower respiratory tract infection aged over 3 years, 90 CAP children were enrolled in the study. Throat swab MP realtime PCR and serum enzyme-linked immunosorbent assays (ELISA) IgM antibodies were performed. A positive rate of MP PCR and serum IgM, the level of IgM index, clinical features, and laboratory findings were analyzed.
Results
MP PCR was positive in 57 cases. Longer fever duration before admission (P<0.001), higher rates of lobar or segmental pneumonia (P=0.048), unilateral infiltration (P=0.038), and extrapulmonary symptoms (P=0.049) were associated with MP PCR-positive pneumonia. Serum IgM index was significantly higher in MP PCR-positive pneumonia them in MP PCR-negative pneumonia (3.9±3.0 vs. 0.8±1.3, P<0.001). Using MP PCR as a gold standard, the sensitivity, specificity, positive predictive value and negative predictive value of serum IgM were 85.5%, 82.1%, 91.4%, and 71.9%, respectively. The area under the curves for serum IgM index was 0.892, and the ROC analysis indicated that an optimal cutoff value of 1.05 for serum IgM provided the highest sensitivity and specificity interestingly (83.9% vs. 85.7%, P<0.001).
REFERENCES
1. Liu WK, Liu Q, Chen DH, Liang HX, Chen XK, Chen MX, et al. Epidemiology of acute respiratory infections in children in Guangzhou: a three- year study. PLoS One. 2014; 9:e96674.
2. He XY, Wang XB, Zhang R, Yuan ZJ, Tan JJ, Peng B, et al. Investigation of Mycoplasma pneumoniae infection in pediatric population from 12,025 cases with respiratory infection. Diagn Microbiol Infect Dis. 2013; 75:22–7.

3. Yang EA, Gang MH, You SY, Kim JH, Lee JH. Clinical characteristics of children with lobar pneumonia caused by Mycoplasma pneumoniae. Pediatr Allergy Respir Dis. 2012; 22:256–64.
4. Park GY, Lee YI, Shin M, Park JO, Kim CH. Clinical differences according to radiological patterns in childhood Mycoplasma pneumoniae pneumonia. Allergy Asthma Respir Dis. 2013; 1:362–9.
6. Izumikawa K, Izumikawa K, Takazono T, Kosai K, Morinaga Y, Nakamura S, et al. Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother. 2014; 20:181–5.
7. Pellan M, Bastian C, Gaudelus J, Delacourt C, de Pontual L. Pulmonary necrotizing cavity caused by Mycoplasma pneumoniae infection. Arch Pediatr. 2013; 20:1158–9.
8. Mishra R, Cano E, Venkatram S, Diaz-Fuentes G. An interesting case of mycoplasma pneumonia associated multisystem involvement and diffuse alveolar hemorrhage. Respir Med Case Rep. 2017; 21:78–81.

9. Izumikawa K. Clinical features of severe or fatal Mycoplasma pneumoniae Pneumonia. Front Microbiol. 2016; 7:800.

10. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae Infection on the basis of possible pathogenesis. Front Microbiol. 2016; 7:23.

11. Topcu Y, Bayram E, Karaoglu P, Yis U, Guleryuz H, Kurul SH. Coexis-tence of myositis, transverse myelitis, and Guillain Barré syndrome following Mycoplasma pneumoniae infection in an adolescent. J Pediatr Neurosci. 2013; 8:59–63.

12. Khoury T, Sviri S, Rmeileh AA, Nubani A, Abutbul A, Hoss S, et al. Increased rates of intensive care unit admission in patients with Mycoplasma pneumoniae: a retrospective study. Clin Microbiol Infect. 2016; 22:711–4.
13. Hon KL, Leung AS, Cheung KL, Fu AC, Chu WC, Ip M, et al. Typical or atypical pneumonia and severe acute respiratory symptoms in PICU. Clin Respir J. 2015; 9:366–71.

14. Morozumi M, Takahashi T, Ubukata K. Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of communi-ty-acquired pneumonia. J Infect Chemother. 2010; 16:78–86.

15. Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. 2017; 60:151–7.

16. Youn YS, Lee SC, Rhim JW, Shin MS, Kang JH, Lee KY. Early additional immune-modulators for Mycoplasma pneumoniae pneumonia in children: an observation study. Infect Chemother. 2014; 46:239–47.
17. Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. 2008; 57:223–8.

18. Yan Y, Wei Y, Jiang W, Hao C. The clinical characteristics of corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Sci Rep. 2016; 6:39929.

19. Qasem JA, Khan ZU, Shiji G, Mustafa AS. Polymerase chain reaction as a sensitive and rapid method for specific detection of Mycoplasma pneumoniae in clinical samples. Microbiol Res. 2002; 157:77–82.

20. Miyashita N, Kawai Y, Kato T, Tanaka T, Akaike H, Teranishi H, et al. Rapid diagnostic method for the identification of Mycoplasma pneumoniae respiratory tract infection. J Infect Chemother. 2016; 22:327–30.

21. Yin YD, Zhao F, Ren LL, Song SF, Liu YM, Zhang JZ, et al. Evaluation of the Japanese Respiratory Society guidelines for the identification of Mycoplasma pneumoniae pneumonia. Respirology. 2012; 17:1131–6.
23. Lee K, Kim WJ, Kim DL, Kim JH, Chong MS. Seroprevalences of Mycoplasma pneumoniae IgM antibodies among children living in Jeju island, Korea. Lab Med Online. 2014; 4:146–51.
24. Miyashita N, Kawai Y, Tanaka T, Akaike H, Teranishi H, Wakabayashi T, et al. Diagnostic sensitivity of a rapid antigen test for the detection of Mycoplasma pneumoniae: comparison with realtime PCR. J Infect Chemother. 2015; 21:473–5.
25. Ferwerda A, Moll HA, de Groot R. Respiratory tract infections by Mycoplasma pneumoniae in children: a review of diagnostic and therapeutic measures. Eur J Pediatr. 2001; 160:483–91.

26. Liu FC, Chen PY, Huang FL, Tsai CR, Lee CY, Lin CF. Do serological tests provide adequate rapid diagnosis of Mycoplasma pneumoniae infection? Jpn J Infect Dis. 2008; 61:397–9.
27. Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol. 1990; 33:253–8.

28. Talkington DF, Shott S, Fallon MT, Schwartz SB, Thacker WL. Analysis of eight commercial enzyme immunoassay tests for detection of antibodies to Mycoplasma pneumoniae in human serum. Clin Diagn Lab Immunol. 2004; 11:862–7.
29. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004; 17:697–728.
30. Nadal D, Bossart W, Zucol F, Steiner F, Berger C, Lips U, et al. Communi-ty-acquired pneumonia in children due to Mycoplasma pneumoniae: diagnostic performance of a seminested 16S rDNA-PCR. Diagn Microbiol Infect Dis. 2001; 39:15–9.
31. Honda J, Yano T, Kusaba M, Yonemitsu J, Kitajima H, Masuoka M, et al. Clinical use of capillary PCR to diagnose Mycoplasma pneumonia. J Clin Microbiol. 2000; 38:1382–4.
32. Kakuya F, Kinebuchi T, Okubo H, Matsuo K. Comparison of oropharyngeal and nasopharyngeal swab specimens for the detection of Mycoplasma pneumoniae in children with lower respiratory tract infection. J Pediatr. 2017; 189:218–21.
33. Reznikov M, Blackmore TK, Finlay-Jones JJ, Gordon DL. Comparison of nasopharyngeal aspirates and throat swab specimens in a polymerase chain reaction-based test for Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis. 1995; 14:58–61.
34. Hsieh SC, Kuo YT, Chern MS, Chen CY, Chan WP, Yu C. Mycoplasma pneumonia: clinical and radiographic features in 39 children. Pediatr Int. 2007; 49:363–7.

35. Wy HH, Min DH, Kim DS, Park MS, Shim JW, Jung HL, et al. Clinical characteristics of Mycoplasma pneumoniae pneumonia in Korean children during the recent 3 epidemics. Allergy Asthma Respir Dis. 2017; 5:8–14.
36. Gao J, Yue B, Li H, Chen R, Wu C, Xiao M. Epidemiology and clinical features of segmental/lobar pattern Mycoplasma pneumoniae pneumonia: a ten-year retrospective clinical study. Exp Ther Med. 2015; 10:2337–44.

37. Waris ME, Toikka P, Saarinen T, Nikkari S, Meurman O, Vainionpää R, et al. Diagnosis of Mycoplasma pneumoniae pneumonia in children. J Clin Microbiol. 1998; 36:3155–9.
38. Thurman KA, Walter ND, Schwartz SB, Mitchell SL, Dillon MT, Baugh-man AL, et al. Comparison of laboratory diagnostic procedures for detection of Mycoplasma pneumoniae in community outbreaks. Clin Infect Dis. 2009; 48:1244–9.
Fig. 1.
Correlation of the fever duration before admission with serum Mycoplasma pneumoniae IgM index in all patients (Pearson correlation coefficient r=0.438, P<0.001).
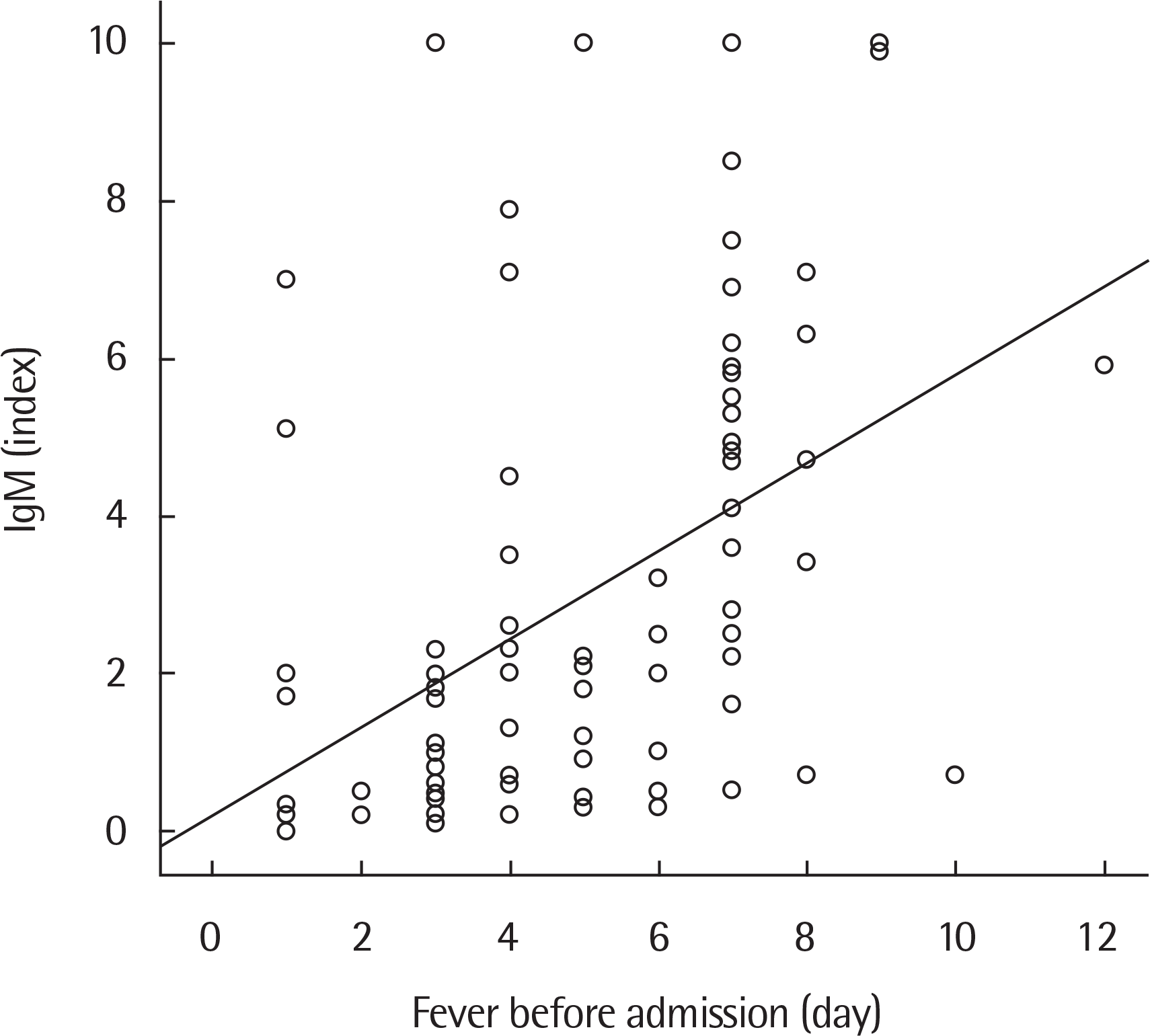
Fig. 2.
Receiver operating characteristic curve for serum M. pneumoniae (MP) IgM index in the diagnosis of MP pneumonia. The optimal cutoff values for serum MP IgM index were 1.05 (area under the curve, 0.892; sensitivity 83.9%, specificity 85.7%; P<0.001).
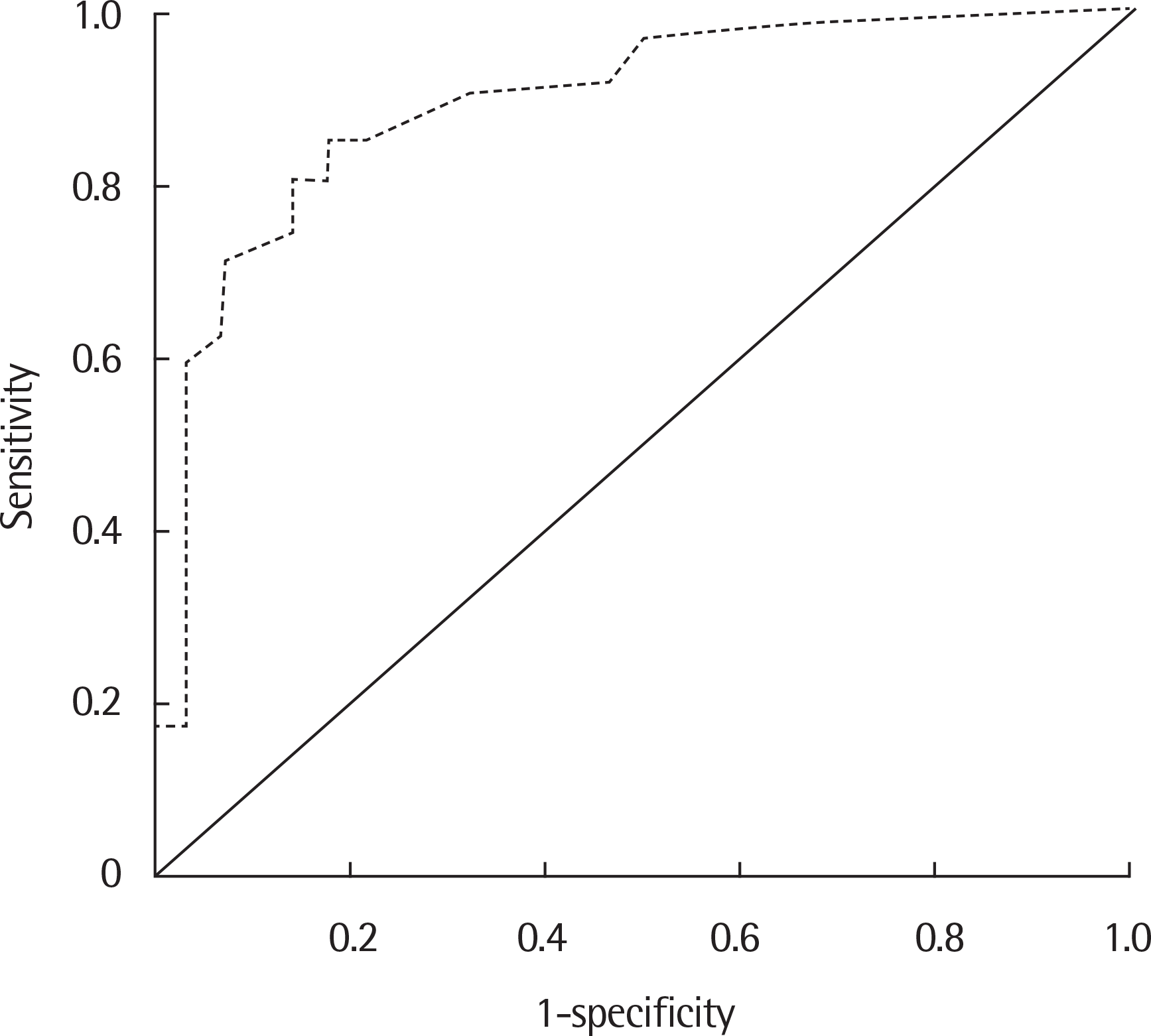
Table 1.
Demographic and clinical features and laboratory findings of study subjects
Table 2.
Relationship between serum Mycoplasma pneumoniae (MP) PCR and IgM antibodies of all study subjects (n=90, P<0.01)
| MP IgM antibodies | MP PCR | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 53 (58.9) | 5 (5.6) | 58 (64.4) |
| Negative | 9 (10.0) | 23 (25.6) | 32 (35.6) |
| Total (n=90) | 62 (68.9) | 28 (31.1) | 90 (100) |
Table 3.
Diagnostic values of serum Mycoplasma pneumoniae (MP) IgM ant bodies with a MP PCR as the gold standard
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | PLR | |
|---|---|---|---|---|---|
| MP IgM antibodies | 85.5 | 82.1 | 91.4 | 71.9 | 4.79 |
Table 4.
Propensity score matching and multivariable Analysis of clinical features and laboratory findings of Mycoplasma pneumoniae PCR positive pneumonia
| Characteristic | Adjusted odds | rattio 95% CI | P-value |
|---|---|---|---|
| Crude analysis | |||
| IgM positivity | 27.09 | 8.80–99.34 | <0.001 |
| Matched analysis∗ | |||
| IgM positivity | 13.50 | 3.69–60.68 | <0.001 |
| Multivariable analysis† | |||
| Fever duration before admission | 1.60 | 1.11–2.31 | 0.013 |
| Unilateral infiltration | 3.50 | 0.90–13.57 | 0.070 |
| Extrapulmonary symptoms | 11.40 | 1.07–121.10 | 0.044 |
| O2 need | 0.12 | 0.01–1.12 | 0.062 |
| Eosinophil (%) | 1.25 | 0.93–1.67 | 0.140 |
| IgM titer (index) | 2.61 | 1.44–4.73 | 0.002 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download