Abstract
Purpose
To compare posture-induced intraocular pressure (IOP) changes in vitrectomized eyes and normal eyes of patients who had vitrectomy in one eye.
Methods
A total of 31 patients older than 20 years of age who underwent vitrectomy were enrolled in the study. At least six months after vitrectomy, we measured IOP in both eyes using a rebound tonometer 10 minutes after the patient assumed sitting, supine, right lateral decubitus, and left lateral decubitus positions. Patients with a history of ocular surgery (not including vitrectomy) or recent medication use associated with IOP were excluded. IOP and ocular parameters of vitrectomized and normal fellow eyes were compared. For the decubitus position, IOP values of dependent and nondependent eyes were compared.
Results
No significant difference was observed in IOP between vitrectomized and normal eyes in the sitting and supine positions. The IOP for dependent eyes (on the lower side in the lateral decubitus position) was significantly higher than the IOP for nondependent eyes in both right lateral decubitus (right vitrectomized eye 19.31 ± 4.20 vs. 16.71 ± 4.02 mmHg, p < 0.001; left vitrectomized eye 18.35 ± 1.75 vs. 16.04 ± 3.02 mmHg, p = 0.003) and left lateral decubitus (right vitrectomized eye 17.32 ± 4.63 vs. 19.15 ± 3.83 mmHg, p = 0.004; left vitrectomized eye 16.19 ± 1.81 vs. 18.12 ± 2.29 mmHg, p < 0.001) positions.
Intraocular pressure (IOP) is a major risk factor for development or progression of glaucoma. IOP is a dynamic parameter that varies with time and environment [123]. Furthermore, it can be affected by intraocular surgery, such as phacoemulsification and vitrectomy [4]. In particular, pars plana vitrectomy is one of the most frequently performed operations for the treatment of vitreous and retinal diseases such as retinal detachment, vitreous hemorrhage, macular hole, and epiretinal membrane. After vitrectomy, most of the vitreous cavity should be filled with aqueous instead of vitreous; considering the different characteristics of aqueous and vitreous, intraocular environments including IOP should not be sustained equally after vitrectomy. Many studies have reported that IOP varies after vitrectomy [567]. Some have indicated that IOP elevation following vitrectomy was observed only in the vitrectomized eye [89]; Lee et al. [10] also suggested that the IOP fluctuation was greater in vitrectomized than in normal eyes. On the other hand, Chang et al. [11] reported that silicone oil-filled eyes showed a peak IOP in the nocturnal period, as observed for the IOP of fellow eyes.
Clinicians have tried to study the dynamic mechanism of IOP by determining the effects of body position on IOP. Some studies have reported that IOP variation and glaucoma severity were dependent on sleep position [1213]. However, no study has investigated the effect of a change in body position on the IOP of vitrectomized eyes. In this study, we investigated posture-induced IOP changes in vitrectomized and normal fellow eyes of patients who underwent vitrectomy in a single eye.
This study was conducted from July to December 2015 and comprised 31 patients who underwent vitrectomy on a single eye at least 6 months before July 2011 to June 2015. This study adhered to the principles outlined in the Declaration of Helsinki for human research, and all participants provided written informed consent. The study protocol was approved by the institutional review board of Kangbuk Samsung Hospital (2015-02-024).
All participants underwent full ophthalmic examinations including slit-lamp examination, Zeiss four-mirror indentation gonioscopy, dilated fundus examination, ultrasound pachymetry (Sonoscan 4000AP; Sonomed, Lake Success, NY, USA) and best-corrected visual acuity. In all cases, patients had undergone vitrectomy on a single eye at least six months prior to the ophthalmic examination. A personal medical interview was used to evaluate ocular diseases, other systemic diseases including hypertension and diabetes, medications, and prior ocular surgeries. Systolic and diastolic blood pressure were measured 10 minutes after arriving at the clinic using an automated noninvasive blood pressure device on the assumption that blood pressure is significantly associated with IOP [14].
Positional IOP data were collected for patients who had one vitrectomized eye and a normal fellow eye. In this study, vitrectomized eye was defined as 1) underwent successful pars plana vitrectomy by a retinal specialist without complications and 2) had no history of antiglaucoma medication use for two months or longer based on previous reports [151617]. Normal fellow eye was defined as 1) open angle eye that was never operated on, 2) no history of antiglaucoma eyedrops for four months, and 3) no ocular pathology or abnormality except cataract on a full ophthalmic examination.
IOP was measured using an Icare rebound tonometer (Icare, Vantaa, Finland) by a single experienced examiner masked to patient characteristics. To obtain reliable data, the examiner obtained six consecutive IOP measurements; the average result was used only when the standard deviation of the measurements was within the normal range. Participants were asked to sit on a chair with back support in a quiet room under dim light. After 10 minutes, IOP was measured in the sitting position. The patient was then asked to lie on a flat bed with a soft pillow underneath the head to maintain a comfortable supine position. After 10 minutes, IOP was measured. Patients were asked to turn right to assume a right lateral decubitus position with the head parallel to the bed on a soft pillow. To minimize the compression effects of the pillow against the eyeball, patients were instructed to put their head near the edge of the pillow. IOP was measured 10 minutes after the positional change to right lateral decubitus. After 10 minutes in a supine position, patients assumed the left lateral decubitus position, and IOP was measured 10 minutes later.
We excluded all patients with history of ocular surgery (except vitrectomy), and we only included phakic patients in this study. Other exclusions were use of antiglaucoma medication within three months, use of steroid medication within three months, peripheral anterior synechia >90 degrees on gonioscopy, and inflammation or anterior chamber reaction. We also excluded patients who underwent vitrectomy with oil tamponade, which affects IOP [18], or who underwent vitrectomy for vitreous hemorrhage from diabetic retinopathy because poor glycemic control may affect IOP [19].
Patients were instructed to stay awake during the positional changes and look straight ahead during IOP measurements. The eye on the lower side in the lateral decubitus position was considered the dependent eye.
We compared positional IOP and ocular parameters of vitrectomized eyes to those of normal fellow eyes. We performed paired t-tests using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA) to analyze differences between vitrectomized and normal fellow eyes. A p-value <0.05 was considered statistically significant.
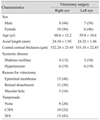
A total of 31 individuals (15 men and 16 women) with one vitrectomized eye and one normal fellow eye were included in the study. The mean patient age was 60.3 ± 14.9 years (range, 21 to 81 years). The mean systolic blood pressure was 124.7 ± 14.4 mmHg (range, 100 to 150 mmHg), and mean diastolic blood pressure was 72.9 ± 9.2 mmHg (range, 55 to 90 mmHg). In addition, 12 patients (38.7%) had hypertension and 7 (22.6%) had diabetes mellitus. Preoperative indications for vitrectomy included epiretinal membrane (15 eyes, 48.4%), retinal detachment (11 eyes, 35.5%), and macular hole (5 eyes, 16.1%). Of the patients, 8 (25.8%) underwent vitrectomy without tamponade, 10 (32.3%) had vitrectomy with C3F8, and 13 (41.9%) with SF6. The mean interval between surgery and IOP measurement was 23.23 ± 14.89 months (range, 7 to 52 months) (Table 1).
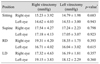
Of the patients, 18 (62.1%) underwent vitrectomy in the right eye. Comparisons of IOP between eyes in different positions are presented in Table 2. No significant differences were observed for IOP in sitting and supine positions. However, IOP of right dependent eyes (19.31 ± 4.20 mmHg) was significantly higher than that of left nondependent eyes (16.71 ± 4.02 mmHg) in the right lateral decubitus position (p < 0.001), and IOP of left dependent eyes (19.15 ± 3.83 mmHg) was significantly higher than that of right nondependent eyes (17.32 ± 4.63 mmHg) in the left lateral decubitus position (p = 0.004). For right vitrectomized eyes, IOP in the right lateral decubitus position (dependent, 19.31 ± 4.20 mmHg) was significantly higher than in the left lateral decubitus position (nondependent, 17.32 ± 4.63 mmHg) (p < 0.001). In left normal fellow eyes, IOP in the left lateral decubitus position (dependent, 19.15 ± 3.83 mmHg) was significantly higher than in the right lateral decubitus position (nondependent, 16.71 ± 4.02 mmHg) (p < 0.001).
The other 13 patients underwent vitrectomy in the left eye. Comparisons of IOP between eyes in different positions are listed in Table 2. No significant differences were observed in IOP in sitting and supine positions. In the right lateral decubitus position, IOP of right dependent eyes (18.35 ± 1.75 mmHg) was significantly higher than that of left nondependent eyes (16.04 ± 3.02 mmHg) (p = 0.003). In the left lateral decubitus position, IOP of left dependent eyes (18.12 ± 2.29 mmHg) was significantly higher than that of right nondependent eyes (16.19 ± 1.81 mmHg) (p < 0.001). In patients with left vitrectomized eyes, IOP in the left lateral decubitus position (dependent, 18.12 ± 2.29 mmHg) was significantly higher than in the right lateral decubitus position (nondependent, 16.04 ± 3.02 mmHg) (p < 0.001). For IOP of right normal fellow eyes in the lateral decubitus position, IOP in the right lateral decubitus position (dependent, 18.35 ± 1.75 mmHg) was significantly higher than that in the left lateral decubitus position (nondependent, 16.19 ± 1.81 mmHg) (p < 0.001).
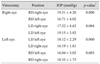
Comparisons of IOP for different positions in 18 patients with right vitrectomized eyes and 13 patients with left vitrectomized eyes are shown in Tables 3 and 4. IOP values of both eyes for different positions in patients with right and left vitrectomized eyes were not significantly different. IOP of right vitrectomized eyes in the right lateral decubitus position (19.31 ± 4.20 mmHg) was not significantly different from that of left vitrectomized eyes in the left lateral decubitus position (18.12 ± 2.29 mmHg) (p = 0.366). IOP of left normal fellow eyes in the left lateral decubitus position (19.15 ± 3.83 mmHg) was not significantly different from that of right normal fellow eyes in the right lateral decubitus position (18.35 ± 1.75 mmHg) (p = 0.440).
Comparisons of IOP measurements in sitting, supine, and lateral decubitus positions are presented in Table 5. IOP was higher for vitrectomized eyes than normal fellow eyes in all positions, but the differences were not significant.
Correlation analysis between the position-induced differences in IOP and the biometric parameters axial length and central corneal thickness is presented in Table 6. No relationship was identified between the position-induced differences in IOP and axial length (r = 0.139, p = 0.483) or IOP and central corneal thickness (r = 0.020, p = 0.921).
IOP is one of the most important factors associated with progression of glaucoma. Therefore, precise evaluation of IOP changes is crucial for postoperative monitoring and management. The effect of vitrectomy surgery on IOP is not yet clear. Fujikawa et al. [9] reported that mean IOP increased after vitrectomy in patients with macular hole. Similarly, Chen [5] reported that, although IOP elevation after stage 3 macular hole surgery was mostly transient and well-controlled by medication, extended medication was needed in some cases. Wu et al. [20] reviewed the IOP of 198 patients who underwent vitrectomy and found that the incidence of high IOP (≥24 mmHg) or increased IOP (≥5 mmHg) was significantly higher in vitrectomized eyes than in control eyes. Moreover, Ki-I et al. [21] reported small IOP changes after phacovitrectomy, with some eyes showing increased IOP over time. In contrast, Yu et al. [22] demonstrated that vitrectomy did not increase the risk of ocular hypertension in 441 vitrectomized patients. That study found that ocular hypertension occurred in 4.31% of vitrectomized eyes and 2.49% of control eyes. Despite a larger proportion of ocular hypertension occurring in vitrectomized eyes, no significant difference was found between the two groups. Mi and Thompson [23] also reported that vitrectomy was not correlated with increased risk of IOP elevation compared with fellow control eyes. Kim et al. [24] demonstrated that vitrectomy by itself or combined vitrectomy with sub-Tenon injection of triamcinolone acetonide did not increase IOP in the long term. Lalezary et al. [25] reported that vitrectomy did not increase IOP even after removal of the crystalline lens.
IOP could be affected after vitrectomy by several possible mechanisms. After vitrectomy, the vitreous cavity is filled with fluid rather than vitreous. This change could affect the structural or physiological stability of the eye, potentially causing acute or chronic changes in IOP; however, this mechanism is not fully understood. Previous studies have indicated that surgical complications including hemorrhage, inflammation, and gas tamponade can cause an increase in IOP [6262728]. However, this IOP elevation can be controlled with antiglaucoma eyedrops [56]. In other cases, late-onset glaucoma may develop [2729]. Chang [30] reported that increased oxidative stress on the trabecular meshwork after vitrectomy resulted in decreased outflow facility that might increase IOP. Fujikawa et al. [9] suggested that inflammatory stress or the physical effects of vitrectomy could cause increased IOP. In contrast to previous studies, we excluded silicone oil tamponade patients. We also excluded patients with vitrectomy associated with vitreous hemorrhage and those with less than six months follow-up since vitrectomy. These relatively strict inclusion criteria could have led to underestimation of IOP rise after vitrectomy. Also, exclusion of severe cases ruled out more complex vitrectomy requiring longer operation times with higher risk of complication, which might have increased the risk of postoperative IOP increase. The mean interval time between surgery and IOP measurement was 23.23 ± 14.89 months (range, 7 to 52 months) in our study, which was relatively short; Magnusson et al. [31] reported that the incidence of late-onset open-angle glaucoma increased with longer follow-up. Lalezary et al. [25] reported that vitrectomy might alter scleral rigidity and affect episcleral venous pressure, which is directly related to IOP. Increased oxygen tension after vitrectomy may cause arterial constriction, which may reduce blood flow to the ciliary body and limit aqueous humor production. The conventional anterior pathways of aqueous flow may be altered without the vitreous body, resulting in a more posterior flow into an empty cavity, readjusting the fluid-IOP dynamic. McNulty et al. [32] concluded that vitrectomy did not seem to increase IOP, and a substantial proportion of eyes in their study had decreased IOP. Furthermore, in a phakic-vitrectomized eye, elevated oxygen level was modulated by antioxidant mechanisms in the lens, which reduces oxidative stress on the trabecular meshwork.
Previous studies have reported that positional changes could affect IOP. Malihi and Sit [33] found that IOP was lowest when the patient was sitting; IOP was elevated in all other head and body positions. The lateral decubitus position might result in a small increase in IOP in the lower eye. Seo et al. [34] demonstrated that low head position increased the IOP of the dependent eye compared with a neutral head position in the lateral decubitus posture. Similarly, Lee et al. [35] reported that a lateral decubitus posture induced increased IOP in dependent eyes in young healthy individuals. To date, there have been no studies on the effects of body position on IOP in vitrectomized eyes. In our study, we did not find significant differences in IOP based on positional changes between vitrectomized and normal fellow eyes. And IOP was higher in dependent compared to nondependent eyes in the lateral decubitus position, regardless of vitrectomy.
The mechanism for IOP changes with body position is not completely understood. According to previous reports, IOP increased following changes from sitting to supine or lateral decubitus position due to hydrostatic effects and increased episcleral venous pressure or alterations in uveoscleral outflow rate [363738]. Choroidal vascular engorgement associated with body fluid redistribution in the supine position is another possible mechanism [39]. Other studies have suggested that gravity and compression of neck vessels might contribute to IOP increase in supine and lateral decubitus positions. This is supported by the finding that both episcleral venous pressure and IOP increased as the angle of the head-down body position increased [40]. Moreover, Friberg and Weinreb [41] found a 1-mmHg increase in IOP for every 0.83 ± 0.21-mmHg increase in episcleral venous pressure in a gravity-inverted human study, associated with the cephalad fluid shift that occurs during complete gravity inversion. However, the effects of postural change on ocular perfusion pressure or the translaminar pressure gradient are still unknown.
De Koninck et al. [42] reported that a preference for side sleeping appears more frequently in elderly adults, and this sleep pattern was more common with age. Given that IOP of dependent eyes was higher in the lateral decubitus posture than in the supine position and IOP of dependent eyes was higher than that of nondependent eyes regardless of vitrectomy, patients with progressive glaucoma in one eye might benefit from sleeping in the supine position or on the contralateral side for IOP control.
Our study has some limitations. First, cross-sectional studies have inherent limitations because they cannot define temporal relationships. We also had a small sample and did not control for preoperative diagnosis. The non-randomized sequence of postural changes may be another limitation. Additionally, adjustment according to the severity of glaucoma could not be performed. Finally, not all systemic parameters that may affect IOP were measured.
In conclusion, in patients who underwent vitrectomy surgery of one eye, IOP of the dependent eye was significantly higher than that of the nondependent eye in both the right lateral decubitus and left lateral decubitus positions, regardless of vitrectomy. No significant difference was observed between vitrectomized and normal fellow eyes, regardless of position. Further studies are needed to investigate the mechanisms and effects of IOP changes according to body position in patients with glaucoma.
Figures and Tables
Table 2
Comparison of positional IOP for vitrectomized and normal fellow eyes of patients with vitrectomy of a single eye

References
1. Ahn JH, Kil HK, Lee MV. Positional change of intraocular pressure and its relationship to ocular pulse amplitude. J Korean Ophthalmol Soc. 2015; 56:234–240.

2. Prata TS, De Moraes CG, Kanadani FN, et al. Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010; 55:445–453.

3. Cheng J, Xiao M, Xu H, et al. Seasonal changes of 24-hour intraocular pressure rhythm in healthy Shanghai population. Medicine (Baltimore). 2016; 95:e4453.

4. Chen PP, Lin SC, Junk AK, et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American academy of ophthalmology. Ophthalmology. 2015; 122:1294–1307.
6. Costarides AP, Alabata P, Bergstrom C. Elevated intraocular pressure following vitreoretinal surgery. Ophthalmol Clin North Am. 2004; 17:507–512.

7. Tranos P, Bhar G, Little B. Postoperative intraocular pressure spikes: the need to treat. Eye (Lond). 2004; 18:673–679.

8. Yamamoto K, Iwase T, Terasaki H. Long-term changes in intraocular pressure after vitrectomy for rhegmatogenous retinal detachment, epi-retinal membrane, or macular hole. PLoS One. 2016; 11:e0167303.

9. Fujikawa M, Sawada O, Kakinoki M, et al. Long-term intraocular pressure changes after vitrectomy for epiretinal membrane and macular hole. Graefes Arch Clin Exp Ophthalmol. 2014; 252:389–393.

10. Lee YW, Kim JM, Shim SH, et al. The influence of a vitrectomy on the diurnal intraocular pressure. J Ophthalmol. 2015; 2015:427808.

11. Chang JH, Shin JP, Kim IT, Park DH. Variation of 24-hour intraocular pressure in silicone oil-filled eyes. Retina. 2016; 36:59–63.

12. Lee TE, Yoo C, Kim YY. Effects of different sleeping postures on intraocular pressure and ocular perfusion pressure in healthy young subjects. Ophthalmology. 2013; 120:1565–1570.

13. Yoo C, Lin S, Na K, et al. Habitual sleeping position and asymmetric structural and functional loss in patients with open angle glaucoma. Acta Ophthalmol. 2015; 93:e593–e595.

14. Xu L, Wang H, Wang Y, Jonas JB. Intraocular pressure correlated with arterial blood pressure: the Beijing Eye Study. Am J Ophthalmol. 2007; 144:461–462.

15. Schlecht LP, Brubaker RF. The effects of withdrawal of timolol in chronically treated glaucoma patients. Ophthalmology. 1988; 95:1212–1216.

16. Stewart WC, Holmes KT, Johnson MA. Washout periods for brimonidine 0.2% and latanoprost 0.005%. Am J Ophthalmol. 2001; 131:798–799.

17. Shah B, Arora V, Wadhwani M, Mishra SK. Prostaglandin analogs. J Curr Glaucoma Pract. 2011; 5:15–20.
18. Matic S, Suic SP, Biuk D, et al. Influence of silicone oil tamponade after vitrectomy on intraocular pressure. Coll Antropol. 2013; 37:Suppl 1. 227–235.
19. Hymowitz MB, Chang D, Feinberg EB, Roy S. Increased intraocular pressure and hyperglycemic level in diabetic patients. PLoS One. 2016; 11:e0151833.

20. Wu L, Berrocal MH, Rodriguez FJ, et al. Intraocular pressure elevation after uncomplicated pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina. 2014; 34:1985–1989.
21. Ki-I Y, Yamashita T, Uemura A, Sakamoto T. Long-term intraocular pressure changes after combined phacoemulsification, intraocular lens implantation, and vitrectomy. Jpn J Ophthalmol. 2013; 57:57–62.

22. Yu AL, Brummeisl W, Schaumberger M, et al. Vitrectomy does not increase the risk of open-angle glaucoma or ocular hypertension: a 5-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1407–1414.
23. Mi CW, Thompson JT. Long-term follow-up of intraocular pressure after vitrectomy in eyes without preexisting glaucoma. Retina. 2015; 35:2543–2551.

24. Kim SY, Shin JA, Yum HR, et al. Long-term trends in intraocular pressure after combined vitrectomy with sub-tenon injection of triamcinolone acetonide. Retina. 2015; 35:564–569.

25. Lalezary M, Kim SJ, Jiramongkolchai K, et al. Long-term trends in intraocular pressure after pars plana vitrectomy. Retina. 2011; 31:679–685.

26. Desai UR, Alhalel AA, Schiffman RM, et al. Intraocular pressure elevation after simple pars plana vitrectomy. Ophthalmology. 1997; 104:781–786.

27. Gedde SJ. Management of glaucoma after retinal detachment surgery. Curr Opin Ophthalmol. 2002; 13:103–109.

28. Han DP, Lewis H, Lambrou FH Jr, et al. Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology. 1989; 96:1357–1362.
29. Honavar SG, Goyal M, Majji AB, et al. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology. 1999; 106:169–176.
30. Chang S. LXII Edward Jackson lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006; 141:1033–1043.

31. Magnusson G, Abrahamsson M, Sjostrand J. Glaucoma following congenital cataract surgery: an 18-year longitudinal follow-up. Acta Ophthalmol Scand. 2000; 78:65–70.

32. McNulty R, Wang H, Mathias RT, et al. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004; 559(Pt 3):883–898.

33. Malihi M, Sit AJ. Effect of head and body position on intraocular pressure. Ophthalmology. 2012; 119:987–991.

34. Seo H, Yoo C, Lee TE, et al. Head position and intraocular pressure in the lateral decubitus position. Optom Vis Sci. 2015; 92:95–101.

35. Lee JY, Yoo C, Jung JH, et al. The effect of lateral decubitus position on intraocular pressure in healthy young subjects. Acta Ophthalmol. 2012; 90:e68–e72.

36. Kim KN, Jeoung JW, Park KH, et al. Effect of lateral decubitus position on intraocular pressure in glaucoma patients with asymmetric visual field loss. Ophthalmology. 2013; 120:731–735.

37. Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol. 1987; 103:523–526.

38. Sultan M, Blondeau P. Episcleral venous pressure in younger and older subjects in the sitting and supine positions. J Glaucoma. 2003; 12:370–373.

39. Smith TJ, Lewis J. Effect of inverted body position intraocular pressure. Am J Ophthalmol. 1985; 99:617–618.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download