This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To evaluate the long-term effects of early postoperative intraocular pressure (IOP) after Ahmed glaucoma valve (AGV) implantation on long-term surgical outcomes.
Methods
This retrospective, non-randomized study included 100 eyes of 100 patients who underwent AGV surgery. We divided the enrolled patients into four groups according to the presence of transient hypotony within the first postoperative week or the presence of a hypertensive phase during the first three postoperative months. Postoperative IOP, the number of glaucoma medications, and cumulative success rate were compared among the groups.
Results
There was significantly better IOP control and a better success rate in the non-hypertensive phase group 2 years postoperatively. However, no significant difference was found in the IOP or success rate at 2 years postoperatively between the transient hypotony and non-hypotony groups. Further subgroup analysis showed that the non-hypotony, non-hypertensive phase group had a significantly higher success rate (100%) at 2 years postoperatively.
Conclusions
We can predict the long-term prognosis after AGV implantation by considering the early postoperative IOP state and the presence of a hypertensive phase.
Go to :

Keywords: Glaucoma, Intraocular pressure, Ahmed valve implantation
Glaucoma drainage devices (GDDs) are an important therapeutic alternative in the management of glaucoma that is resistant to medical therapy and glaucoma filtration surgery [
1]. Their use has increased, particularly in patients whose previous filtering procedures, with or without adjunctive anti-metabolites, failed or were expected to have a very low chance of success [
234]. Various aqueous shunting devices have been developed, including open-tube and valved designs. Hypotony during the immediate postoperative period is a common complication associated with open-tube implants [
56].
The Ahmed glaucoma valve (AGV; New World Medical, Rancho Cucamonga, CA, USA) is a shunt device with a flow-restriction mechanism that is used in refractory glaucoma either as a primary surgical option or after the failure of conventional filtration procedures. It directs the flow of aqueous through the silicone tube and between two thin silicone elastomer membranes in a tapered chamber [
47]. Clinical experience with this implant had indicated that hypotony during the immediate postoperative period is less common than that with other GDDs [
89]. Early postoperative hypotony occurs in the first 1 to 2 weeks after surgery and is defined as an intraocular pressure (IOP) less than or equal to 6 mmHg [
10]. However, good IOP control in the early postoperative period is frequently followed by a large rise in IOP [
1112]. This hypertensive phase, defined as an IOP greater than 21 mmHg within 3 months of surgery, has also been reported after non-valved drainage shunts [
1314]. However, there is little published information on the long-term prognosis according to the early postoperative IOP. A recent report showed that eyes without a hypertensive phase after AGV implantation had a higher success rate [
15].
Therefore, we investigated the effects of early postoperative hypotony and the presence of a hypertensive phase after AGV implantation on surgical prognosis after 2 years.
Materials and Methods
A retrospective chart review examined consecutive patients who underwent placement of an AGV in the glaucoma division of Seoul St. Mary's Hospital between January 2003 and December 2009. All eyes followed for at least 2 years postoperatively were included. This study adhered to the Declaration of Helsinki and approved by the institutional review board of Seoul St. Mary's Hospital (KC15RISI0427). Informed consent was obtained from each patient.
Preoperative data were collected from the patient records and included age at the time of the surgery, gender, eye laterality, diagnosis, mean IOP during the 3 months before the surgery, the number of medications being taken during the 3 months before the surgery, and mean deviation of the Humphrey visual field (24-2 full-threshold or Swedish interactive threshold algorithm). We excluded any patients with an IOP exceeding 21 mmHg on the first postoperative day or those with an IOP of less than 6 mmHg after one postoperative week; those who required additional glaucoma surgery due to an IOP increase or surgical complication; those who required anterior chamber reformation due to hypotony that caused flat anterior chamber for more than 1 week or kissing choroidal detachment; or those who had undergone another ophthalmologic intraocular surgery (i.e., cataract surgery or retinal surgery). When both eyes were eligible, we randomly choose one eye for inclusion.
All surgeries were performed by one of the authors (CKP). During the procedure, the eye was prepared and draped after retrobulbar anesthesia. A lid speculum was situated and an 8-0 Vicryl (Ethicon, Irvine, CA, USA) suture was placed in the superior midstromal cornea and used for downward retraction. A limbal-based flap of the conjunctiva and Tenon capsule was created in the superior temporal quadrant. The AGV was positioned in the middle of the quadrant, with the anterior edge of the plate at a position of at least 8 mm posterior to the superior temporal corneoscleral limbus. Before implantation, the AGV was tested and primed using a balanced salt solution. The tube was trimmed to extend 1 to 2 mm beyond the limbus. Parallel to the iris plane, a 23-gauge needle was used to enter the anterior chamber at the limbus. Then, the tube was inserted into the anterior chamber through this needle track and secured to the sclera with one 10-0 nylon suture. Partial ligation of the tube was performed using 9-0 nylon sutures. A scleral patch graft was prepared and sutured to cover the drainage tube with four interrupted 10-0 nylon sutures. The conjunctiva and Tenon capsule were reapproximated to the limbus with 8-0 Vicryl sutures.
Postoperative data were collected from the patient records for all consecutive visits. Data collected included IOP measurements, number of medications used, surgical complications, and follow-up duration. Visual fields mean deviation data were collected from up to 1 to 2 years after surgery when available. The hypertensive phase was defined as an IOP >21 mmHg during the first 3 months after surgery with or without any antiglaucoma medication and not caused by tube obstruction, tube retraction, or valve malfunction. Based on the presence of a hypertensive phase, the patients were divided into a hypertensive group and non-hypertensive group. When IOP exceeded 30 mmHg during the hypertensive phase, antihypertensive medication was given with respect to the patient's condition. Hypotony was defined as when the IOP was less than 6 mmHg without glaucoma medication within 2 weeks postoperatively and when it was without cause by wound site leakage or valve malfunction. Based on the presence of hypotony, the patients were also divided into a hypotony group and non-hypotony group. Patients were classified into four groups according to the presence of early hypotony and hypertensive phase; eyes with transient early hypotony and a hypertensive phase (group I), eyes with early hypotony but without a hypertensive phase (group II), eyes without early hypotony but with a hypertensive phase (group III), and eyes without both hypotony and a hypertensive phase (group IV).
The main outcome measure was surgical success, defined as an IOP of less than 21 mmHg without additional glaucoma surgery. Another lower cutoff criterion of success was an IOP of less than 18 mmHg. Failure occurred when the IOP exceeded the success range on two consecutive visits. Secondary outcome measures were the IOP level and number of medications at 1 day; 1 week; and 1, 3, 6, 12, and 24 months after surgery.
The baseline characteristics of the groups were compared using an independent Student's t-test or one-way analysis of variance. The chi-squared test was used to compare categorical data. Kaplan-Meier survival analysis and the logrank test were used to compare the cumulative risk ratio of surgical failure. Statistical analysis was performed using the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered to be statistically significant.
Go to :

Results
The records for 108 eyes (of 108 patients) were examined. Eight eyes were excluded because two eyes underwent additional procedures due to low IOP in the early postoperative period and six eyes underwent anterior chamber reformation due to hypotony resulting from f lat anterior chamber or kissing choroidal detachment. The remaining 100 eyes (in 100 patients) were further analyzed. The preoperative IOP was 31.9 ± 8.0 mmHg and the total number of preoperative medications was 2.8 ± 2.1. The mean follow-up duration was 31.3 ± 0.9 months. Other baseline characteristics are described in
Table 1.
Table 1
Characteristics of the patients
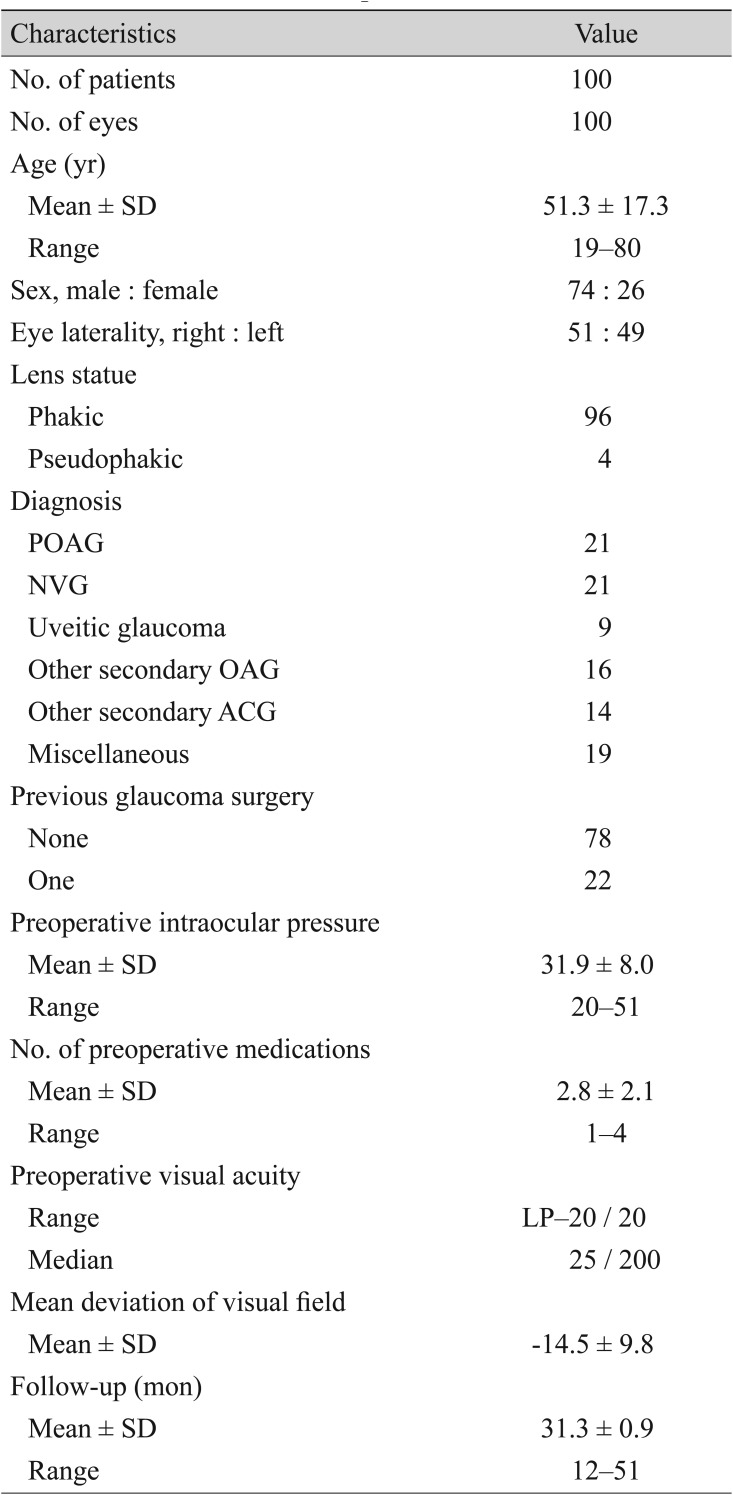

Table 2 compares the preoperative characteristics between the hypertensive and non-hypertensive groups. There were no significant differences with respect to age, preoperative IOP, number of preoperative medications, or mean deviation of the visual field between the two groups. The mean postoperative IOPs of the hypertensive and non-hypertensive groups are plotted in
Fig. 1. IOP differed significantly between the two groups at 1, 3, 6, 12, and 24 months postoperatively (
p < 0.01,
p < 0.01,
p < 0.01,
p < 0.01, and
p < 0.01, respectively). The postoperative number of medications was 2.9 ± 0.8 for the hypertensive group and 1.9 ± 0.4 for the non-hypertensive group and differed significantly between the two (
p = 0.03). The success rate at 2 years postoperation was significantly higher in the non-hypertensive group than in the hypertensive group (95.0% and 72.6%, respectively;
p = 0.02). The success rate was also significantly different between in the non-hypertensive and hypertensive groups when the success was defined as an IOP of less than 18 mmHg (96.20% and 72.6%, respectively;
p = 0.02). The success rate was significantly different between the non-hypertensive group and the hypertensive group (83.8% and 65.4%, respectively;
p = 0.04) when it was defined as an IOP of less than 18 mmHg.
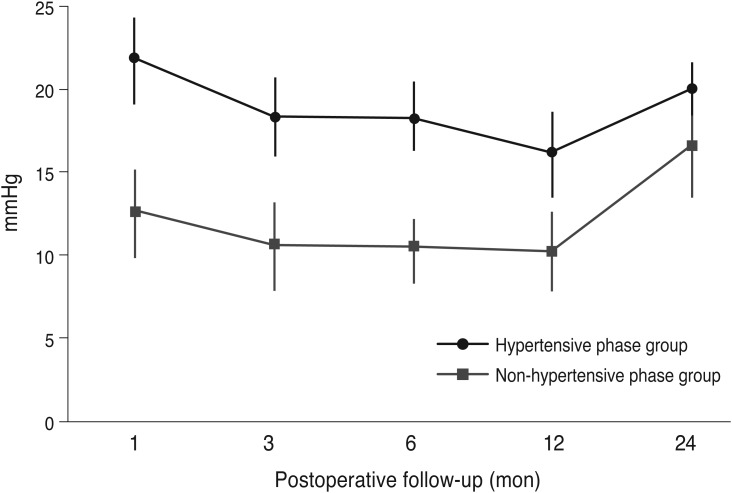 | Fig. 1A comparison of intraocular pressure IOP between the hypertensive phase and non-hypertensive phase groups.
|
Table 2
A comparison of preoperative data between the hypertensive phase group and non-hypertensive phase group
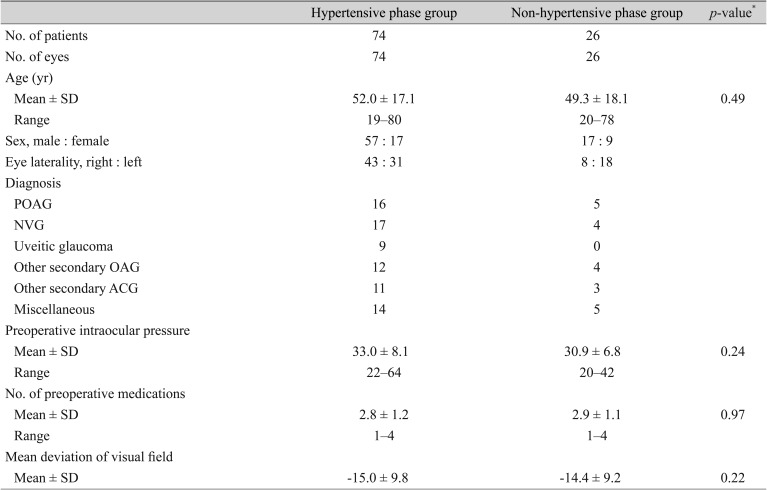

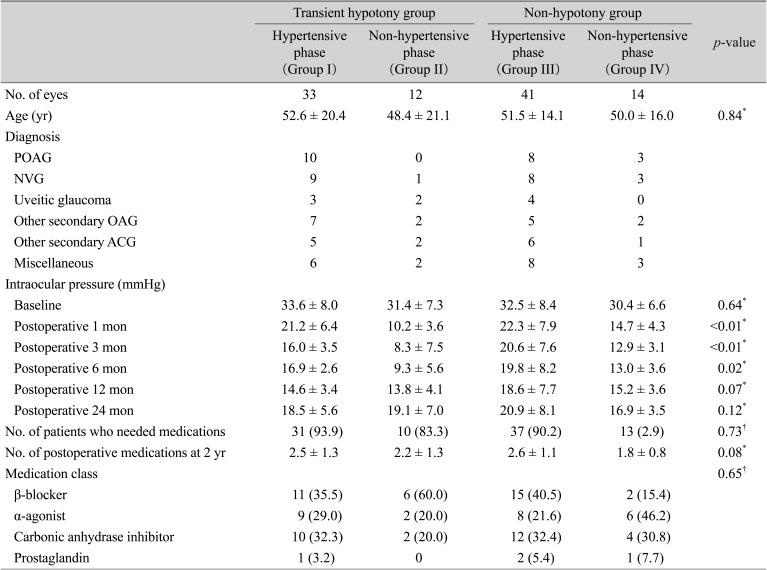
Table 3 compares the preoperative data between the hypotony and non-hypotony groups. The baseline characteristics of the two groups did not differ statistically. The mean IOP did differ significantly between the two groups at 3 and 6 months postoperatively (
p < 0.01 and
p = 0.02, respectively), but not at 12 months and 24 months postoperatively (
p = 0.33 and
p = 0.74, respectively). The mean postoperative IOPs of the hypotony and non-hypotony groups are plotted in
Fig. 2. There was also no significant difference in the number of postoperative medications (2.6 ± 1.1 and 2.4 ± 0.9, respectively) or 2-year success rate (hypotony group 79.4%, non-hypotony group 78.6%,
p = 0.84) between the groups. The success rate was not different between the non-hypertensive group and the hypertensive group (61.8% and 55.6%, respectively;
p = 0.33) when it was defined as an IOP of less than 18 mmHg.
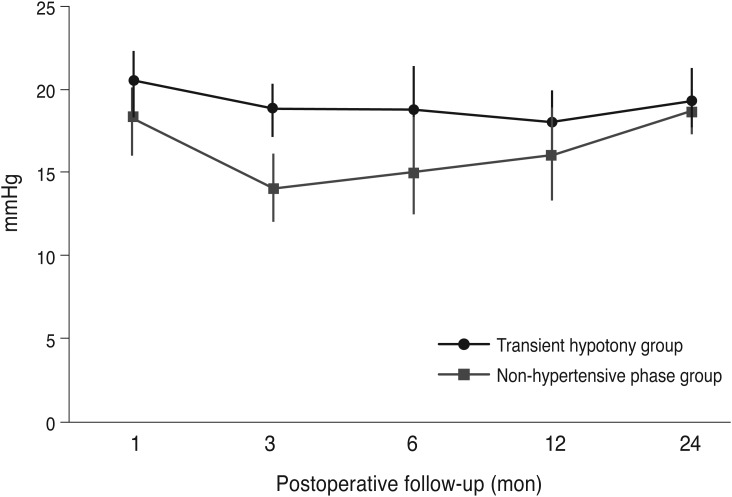 | Fig. 2A comparison of intraocular pressure between the transient hypotony and non-hypotony groups.
|
Table 3
A comparison of preoperative data between the transient hypotony group and non-hypotony group
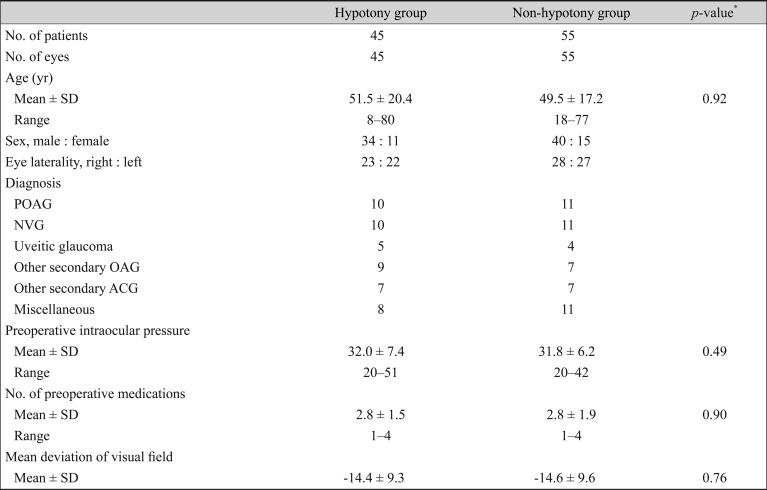

When the patients were divided into four groups according to the presence of both a hypertensive phase and hypotony, the mean postoperative IOP during the first 12 postoperative months was significantly lower in eyes with transient early hypotony but without a hypertensive phase (group II) and significantly higher in eyes without early hypotony but with a hypertensive phase (group III). However, the postoperative IOP at 24 months and the number of postoperative medications did not differ significantly among the four groups (
Table 4). Group III (non-hypotony and hypertensive phase group) had the lowest success survival rate and group IV (non-hypotony and non-hypertensive phase group) had the highest success survival rate in the Kaplan-Meier analysis (log-rank test,
p = 0.03) (
Fig. 3).
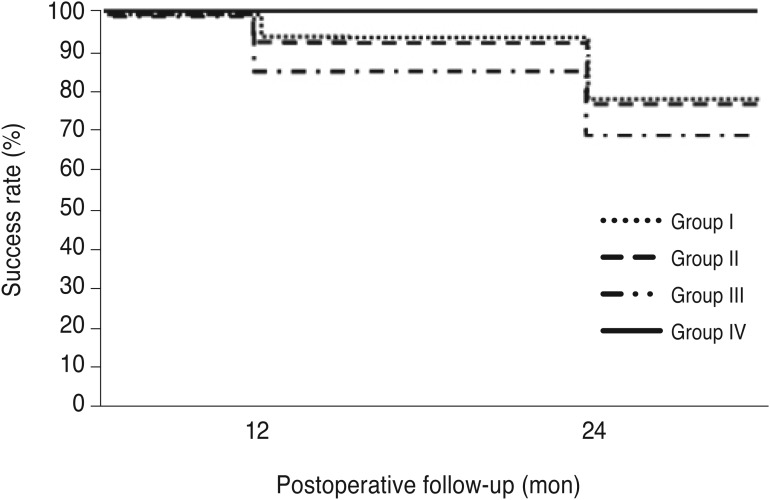 | Fig. 3Kaplan-Meier cumulative probability curve of the success rate in the groups according to the presence of early postoperative transient hypotony and a hypertensive phase. Group I, transient hypotony with a hypertensive phase; group II, transient hypotony without a hypertensive phase; group III, non-hypotony with a hypertensive phase; group IV, non-hypotony without a hypertensive phase.
|
Table 4
A comparison of the four groups according to presence or absence of transient hypotony and hypertensive phase

Go to :

Discussion
A hypertensive phase is one of the early findings after AGV implantation. Huang et al. [
9] reported the presence of a higher mean IOP 2 months after insertion of an AGV implant as compared with at 1 and 2 years after surgery. Ayyala et al. [
11] evaluated 85 patients who underwent insertion of an AGV for the control of refractory glaucoma. Based on the criterion IOP >21 mmHg over the first 6 months after surgery, they reported a hypertensive phase in 70 patients (82%). The IOP peaked at 1 month and stabilized at 6 months. Nouri-Mahdavi and Caprioli [
12] reported that a hypertensive phase occurred in 56% of the eyes studied a s d efined using a c riterion of IOP >21 mmHg during the first 3 months after surgery because they believed that eyes showing an increase in IOP after 3 months postoperatively might be demonstrating a poor IOP outcome rather than a hypertensive phase. Yuen et al. [
16] performed a subanalysis comparing mean IOP at 3 months between a group that had a hypertensive phase and one that did not. The hypertensive group had a significantly higher mean IOP and more uncontrolled IOP as compared with the non-hypertensive group.
In our study, based on the definition used by Nouri-Mahdavi and Caprioli [
12], a hypertensive phase occurred in 74% of the eyes studied. This is higher than in their reports. The identified risk factors for IOP elevation after trabeculectomy are related with the level of inflammation. Patients who experience an IOP spike after trabeculectomy had a significantly higher untreated IOP at 3 and 5 years after surgery. Our results may also be related with the inflammation and fibrosis that occurs following AGV implantation. We observed a significantly lower success rate in the hypertensive phase group at 2 years after AVG implantation. As reported earlier, our results show that it is difficult to control IOP in eyes experiencing a hypertensive phase [
1216].
To our knowledge, no published report explains why a hypertensive phase occurs after AGV insertion. Molteno and Dempster [
17] described the sequential wound healing that occurs after insertion of a single-plate Molteno implant and IOP elevation. After a short hypotensive phase lasting between seven and 10 days postoperatively, the IOP increased gradually in accompaniment with the formation of a well-circumscribed bleb over the AGV reservoir. During the first few weeks after the hypotensive phase, there is intense congestion of the bleb, with the untreated IOP rising from 30 to 50 mmHg. With a reduction in bleb congestion and inflammation over the ensuing months, the bleb becomes less dense and the IOP stabilizes. Nouri-Mahdavi and Caprioli [
12] postulated that the sequence of events with the AGV was likely similar and that the hypertensive phase could probably be explained by the congestion, edema, and fibrosis of the conjunctiva above and around the plate of the GDD. Another possible explanation is the plate size of the AGV. In the single-plate Molteno implant, which has a smaller surface area than that of the AGV, a more pronounced elevation of IOP may occur after implantation [
1819]. In comparison, patients with Baerveldt implants did not have a hypertensive phase because the Baerveldt device has a larger plate surface area than that of the AGV [
5]. The 1-year outcomes of the Ahmed-Baerveldt comparison study show that the Baerveldt implant has a significantly lower mean IOP and a higher success rate as compared with the AGV implant [
20]. It is strongly suggested that the presence of a hypertensive phase could be related with long-term IOP control and its success rate. Recently, there have been reports showing that early use of aqueous suppressants may prevent a hypertensive phase and are related with better outcomes after drainage device surgery [
2122]. There were also patients who used aqueous suppressants after surgery in our study; however, we could not perform a comparison to find out the effect of these medications in terms of surgical outcomes related with the presence of a hypertensive phase.
In our study, the outcome differed according to the presence of transient hypotony after AGV implantation. The reported incidence of early postoperative hypotony ranges between 8% [
9] and 26% [
23] for uveitic glaucoma patients. Other studies have reported that it occurs in 42% of uveitic glaucoma patients [
242526]. In our study, 45% of the eyes showed transient hypotony within 2 weeks postoperation. Of these, 42 eyes (93%) showed hypotony at 1 day postoperatively and three eyes (7%) showed it at 5 days postoperatively. However, the IOP of all patients was above 6 mmHg at 1 week postoperatively without the use of additional medications. In the uveitic glaucoma patients, transient hypotony appeared in five eyes (56%). This result is similar to those seen in previous studies. The presence of hypotony was not significantly related with age, gender, diagnosis, or preoperative IOP in a multiple regression analysis (data not shown).
There are no known reports on long-term prognosis in patients with transient hypotony. In our study, transient hypotony, which occurs in the first 2 weeks postoperatively, did not seem to influence the long-term success of AGV implantation (79.4% in the hypotony group vs. 78.6% in the non-hypotony group at 2 years postoperation). However, postoperative IOP control was better in group II (transient hypotony with non-hypertensive phase group) until 12 months postoperatively, as shown in
Table 4. If we had analyzed the success rate at 6 months postoperatively, there might have been a difference according to the presence of early hypotony. In addition, group II used fewer glaucoma medications during the low IOP period as compared with the other three groups. Based on the postoperative aqueous outflow model of Kotliar et al. [
27], the lower the postoperative IOP, the less the aqueous humor uses the natural outflow pathways, including the trabecular meshwork. More study is needed to determine the exact mechanism, although using the natural outflow pathways could be an advantage in IOP control after AGV implantation in eyes with early postoperative hypotony.
This study was limited by its small number of cases and retrospective design because of the lack of other information about glaucoma surgery prognosis. Another limitation was that we could not assess the histology of the fibrous capsule. Patients with only transient early hypotony that resolved spontaneously were included in this study. Patients with hypotony who underwent additional procedures were excluded from this study and this should be considered when interpreting our results.
In conclusion, a hypertensive phase after AGV implantation significantly influences the long-term IOP and success rate. Transient hypotony, which does not require additional surgery and resolves within 1 week, may also result in better IOP control in the first 12 months postoperatively. The early IOP state can predict the long-term IOP control and prognosis after AGV implantation.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download