Abstract
Placental chorioangioma is the most common non-trophoblastic and hamartoma-like tumor and has generally good prognosis if the size is small. The incidence of a placental chorioangioma is an estimated 1% of all deliveries. The size of a chorioangioma is considered significant if it is larger than 4 cm, since fetal compromise can occur due to circulatory overload. Very rarely, a placental chorioangioma can directly exert fetal circulation to cause fetal cerebral ischemic stroke, especially in giant placental chorioangiomas which are defined as more than 4-5 cm in diameter. Here, we report a case of a huge chorioangioma, sized 7×5 cm with thrombo-occlusion in the placenta associated with neonatal stroke. Perinatal stroke and giant placental chorioangioma are each very rare. Moreover, the combination is extremely rare as is expected. Our case implicates that placental examination should be considered as an important diagnostic workup in cases of perinatal stroke with unknown etiology.
REFERENCES
1). Amer HZ., Heller DS. Chorangioma and related vascular lesions of the placenta—a review. Fetal Pediatr Pathol. 2010. 29:199–206.
2). Kodandapani S., Shreshta A., Ramkumar V., Rao L. Chorioangioma of placenta: a rare placental cause for adverse fetal outcome. Case Rep Obstet Gynecol. 2012. 2012:913878.

3). Wu Z., Hu W. Clinical analysis of 26 patients with histologically proven placental chorioangiomas. Eur J Obstet Gynecol Reprod Biol. 2016. 199:156–63.

4). Mineyko A., Kirton A. The black box of perinatal ischemic stroke pathogenesis. J Child Neurol. 2011. 26:1154–62.

5). Zanardini C., Papageorghiou A., Bhide A., Thilaganathan B. Giant placental chorioangioma: natural history and pregnancy outcome. Ultrasound Obstet Gynecol. 2010. 35:332–6.

6). Li C., Miao JK., Xu Y., Hua YY., Ma Q., Zhou LL, et al. Prenatal, perinatal and neonatal risk factors for perinatal arterial ischaemic stroke: a systematic review and meta-analysis. Eur J Neurol. 2017. 24:1006–15.

7). Deeg KH. Sonographic and Doppler sonographic diagnosis of neonatal ischemic stroke. Ultraschall Med. 2017. 38:360–76.
8). Ghidini A., Locatelli A. Diffuse placental chorioangiomatosis causing multiple fetal cerebral embolism: a case report. J Reprod Med. 2006. 51:321–4.
9). Al Wattar BH., Hillman SC., Marton T., Foster K., Kilby MD. Placenta chorioangioma: a rare case and systematic review of literature. J Matern Fetal Neonatal Med. 2014. 27:1055–63.

10). Zanardini C., Papageorghiou A., Bhide A., Thilaganathan B. Giant placental chorioangioma: natural history and pregnancy outcome. Ultrasound Obstet Gynecol. 2010. 35:332–6.

11). Das S., Ankola P., Chiechi M., Sandhu J. Perinatal cerebral arterial infarction associated with a placental chorioangioma. Am J Perinatol. 2008. 25:381–3.

12). Roh CR. Ischemic perinatal stroke. Korean J Obstet Gynecol. 2009. 52:391–7.
Fig. 1
(A) In ultrasonographic examination at 36 weeks of gestation, a 7×5 cm sized well circumscribed, round, heterogeneous mixed echoic mass along the fetal surface of the placenta was seen. (B) It looks chorioangioma and with color Doppler sonography increased blood flow was seen along the hypoechoic portion of mass. Giant chorioangioma measuring more than 4 cm has a risk of shunting of blood flow from fetus to mass.
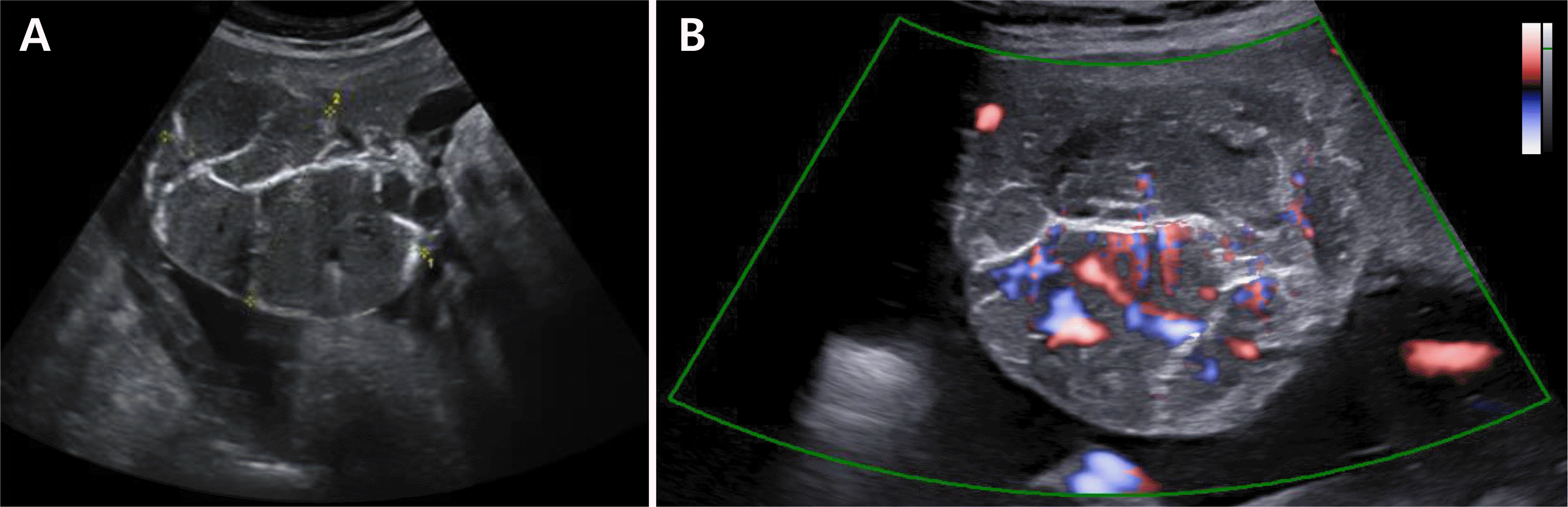
Fig. 2
(A) The brain ultrasonography taken at the day after birth showed asymmetrical ventricles with irregular choroid plexus contour, otherwise no abnormal findings associated with this event. (B) Seven days after birth, brain magnetic resonance imaging was done and the arrow indicates acute infarction in left temporo-parieto-occipital area (arrows) that supposed venous infarcts due to superior sagittal sinus thrombus or arterial infarct on posterior division of middle cerebral artery territory.
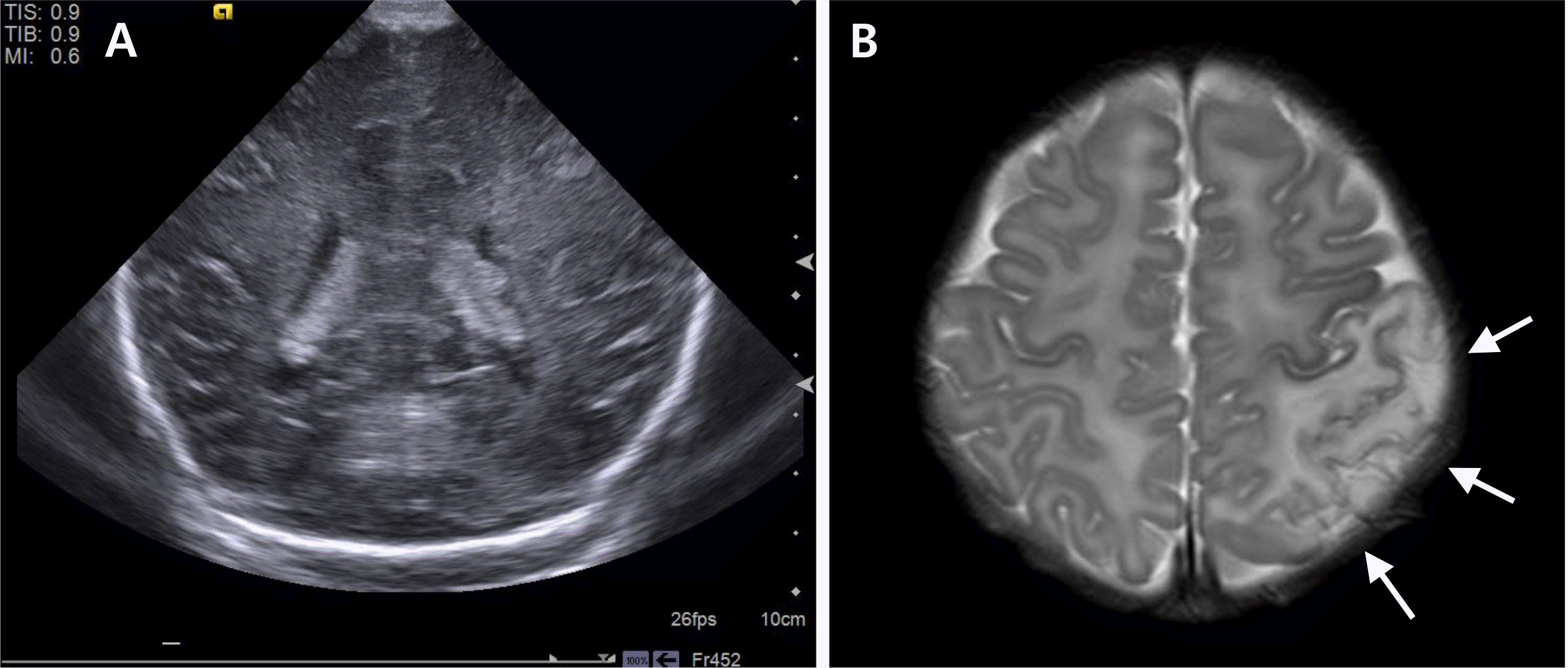
Fig. 3
Pathological findings of the placental mass. (A) A lobulated solid mass (arrows) is present at the periphery of the placental disc. (B) The cut surface of the mass is pinkish brown with multi focal tan brown or yellow degenerating areas. (C) Microscopically, the mass is composed of pro liferating capillaries (lower area) with multifocal infarction (upper left area). The adjacent large vessels show fibrin deposition in the wall with early thrombi (arrows). (D) The magnified image of the inset in (C). Original magnification in (C) 10×, (D) 50×.
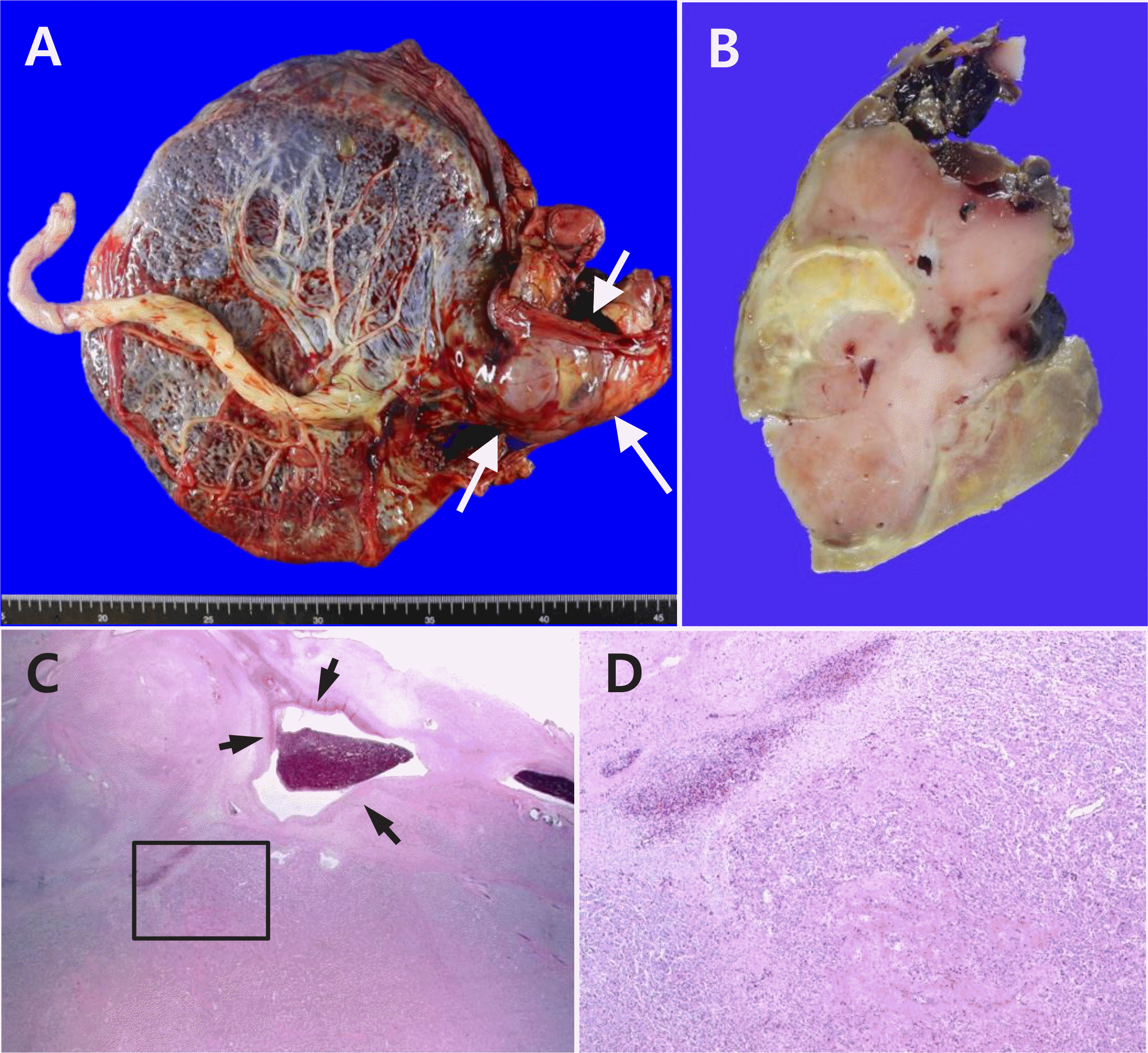
Fig. 4
(A) Two months after birth, brain magnetic resonance (MR) imaging and MR angiography follow up was done and showed attenuation of left distal middle cerebral artery branch due to previous arterial obstruction or secondary changes after infarction. (B) Progression of encephalomalacia of the left parietal lobe was seen.
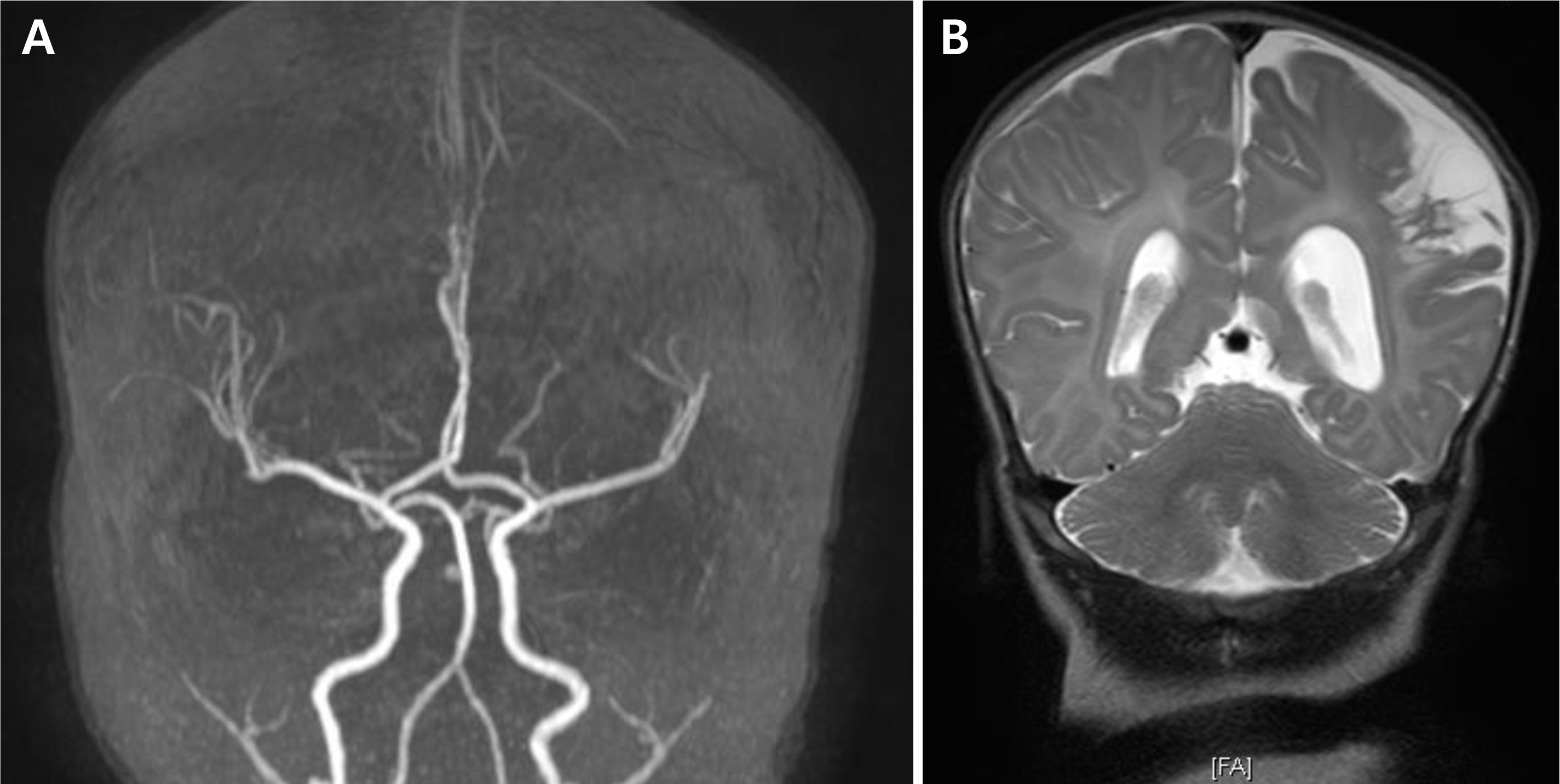




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download