Abstract
Objective
The aim of this study was to analyze the relationship between the circulating adiponectin levels and osteoarthritis (OA).
Methods
Meta-analysis was conducted on the serum/plasma adiponectin levels in patients with OA and controls, and subgroup analysis was performed based on ethnicity, sex, OA site, and adjustment for age, sex, and/or body mass index (BMI).
Results
Seven studies with eleven comparisons including 538 patients with OA and 366 controls were selected, which showed that the adiponectin levels were significantly higher in the OA group than in controls (standardized mean difference [SMD]=0.745, 95% confidence interval [CI]=0.316∼1.174, p=0.001). Stratification according to ethnicity showed significantly elevated adiponectin levels in Caucasian individuals with OA (SMD=0.769, 95% CI=0.218∼1.319, p=0.006). Stratification according to sex revealed significantly higher adiponectin levels in women with OA, but not in men (SMD=0.861, 95% CI=0.099∼1.623, p=0.027; SMD=1.177, 95% CI=−0.911∼3.316, p=0.281). Stratification by an adjustment for age, sex, or BMI showed significantly higher adiponectin levels in the OA group (SMD=0.837, 95% CI=0.326∼1.349, p=0.001). Stratification according to the OA site showed significantly higher adiponectin levels in patients with knee OA (SMD=0.938, 95% CI=0.456∼1.419, p<0.001).
Go to : 
REFERENCES
1. Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999; 107:542–8.
2. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013; 21:16–21.

3. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013; 9:191–200.
4. Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012; 11:8–20.

5. Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces proinflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012; 287:36896–904.

6. Lee YA, Ji HI, Lee SH, Hong SJ, Yang HI, Chul Yoo M, et al. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: implications for arthritic joints. Exp Mol Med. 2014; 46:e72.

7. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003; 112:91–100.

8. Toussirot E, Michel F, Béreau M, Dehecq B, Gaugler B, Wendling D, et al. Serum adipokines, adipose tissue measurements and metabolic parameters in patients with advanced radiographic knee osteoarthritis. Clin Rheumatol. 2017; 36:2531–9.

9. Tootsi K, Kals J, Zilmer M, Paapstel K, Märtson A. Severity of osteoarthritis is associated with increased arterial stiffness. Int J Rheumatol. 2016; 2016; 6402963.

10. Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010; 41:593–8.

11. Cuzdan Coskun N, Ay S, Evcik FD, Oztuna D. Adiponectin: is it a biomarker for assessing the disease severity in knee osteoarthritis patients? Int J Rheum Dis. 2017; 20:1942–9.

12. Laurberg TB, Frystyk J, Ellingsen T, Hansen IT, Jørgensen A, Tarp U, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J Rheumatol. 2009; 36:1885–91.

13. Filková M, Lisková M, Hulejová H, Haluzík M, Gatterová J, Pavelková A, et al. Increased serum adiponectin levels in female patients with erosive compared with non-erosive osteoarthritis. Ann Rheum Dis. 2009; 68:295–6.
14. de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, et al. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012; 20:846–53.

15. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.

16. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13.

17. Wells GA, Shea B, O'connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa (ON): The Ottawa Hospital Research Institute;2000. Available from:. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
18. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed.New York: Psychology Press;2009.
19. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997; 315:1533–7.

21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.

22. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

23. Duval S, Tweedie R. Trim and fill: A simple fun-nel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–63.

24. Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011; 13:R184.

25. Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006; 1762; 711–8.

26. Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004; 323:630–5.

27. Haugen F, Drevon CA. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology. 2007; 148:5478–86.
Go to : 
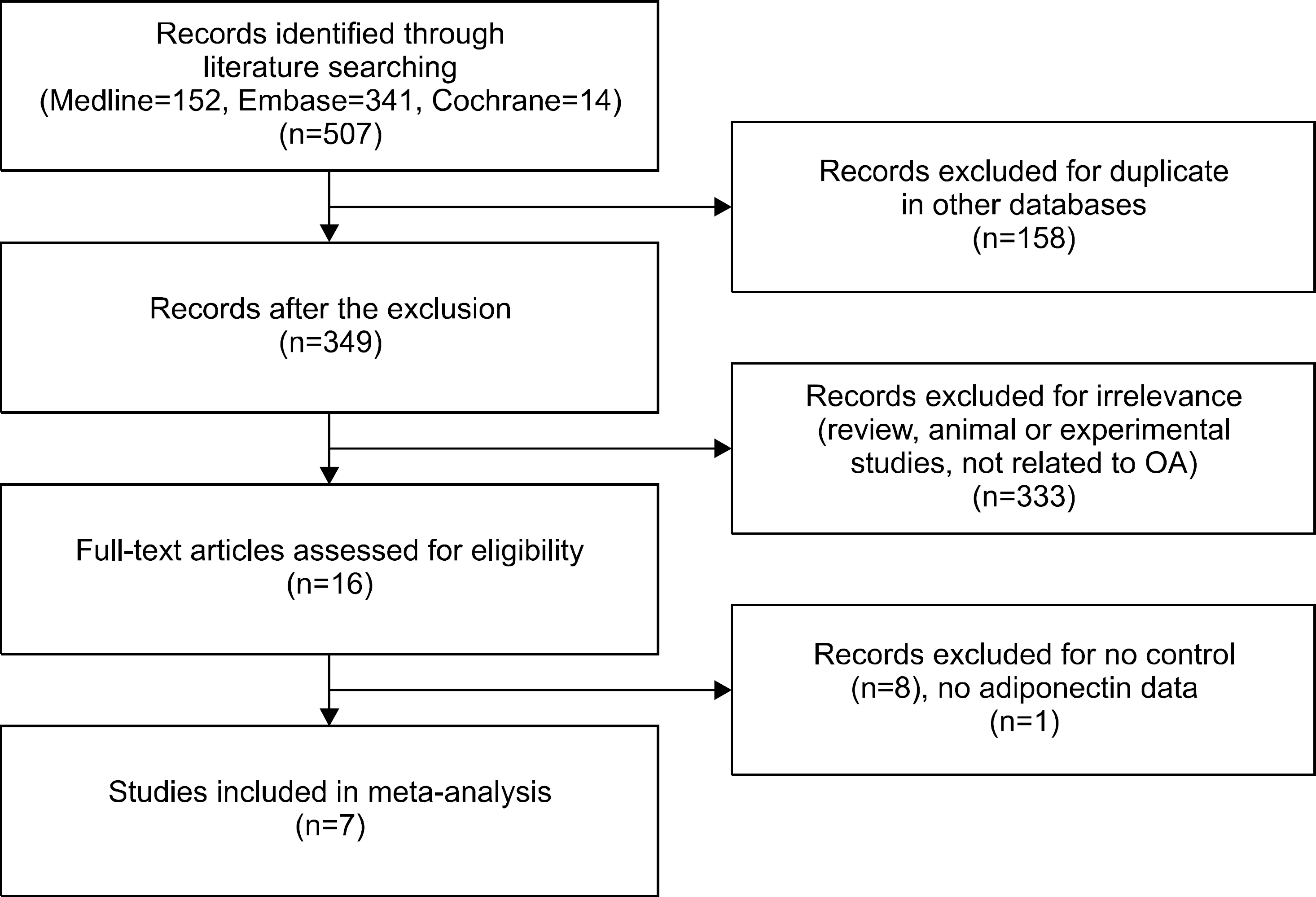 | Figure 2.Meta-analysis of the relationship between adiponectin levels and osteoarthritis (OA). Std diff: standard difference, CI: confidence interval. |
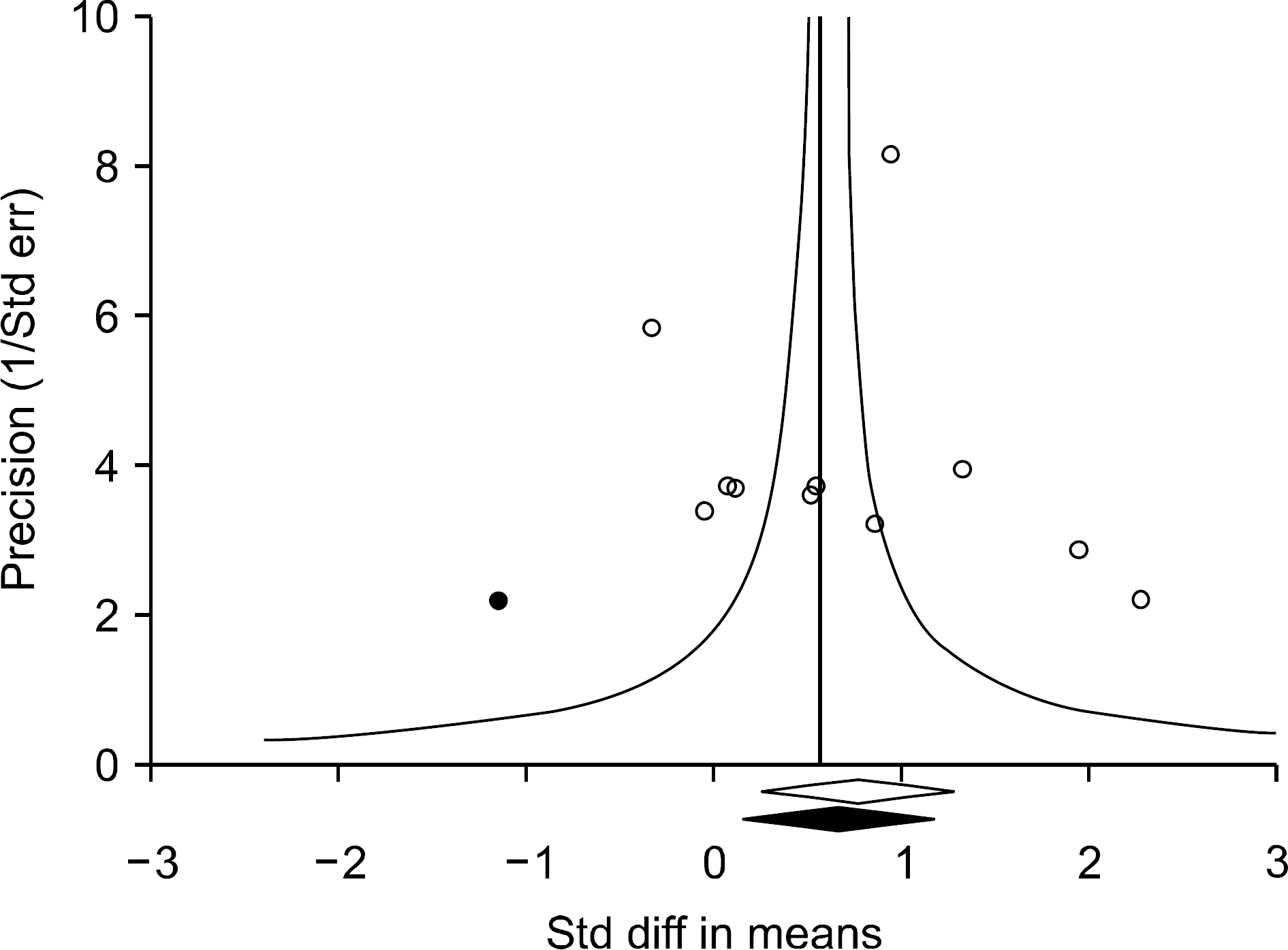 | Figure 3.Funnel plot of studies that examined the association between adiponectin levels and osteoarthritis (Egger's regression p-value=0.559). The filled circles represent studies that showed publication bias. The diamonds at the bottom of the figure show summary effect estimates before (open) and after (filled) publication bias adjustment. Std diff: standard difference, Std err: standard error. |
Table 1.
Characteristics of individual studies included in the meta-analysis
| Author | Country | Ethnicity | Patient | Age (yr) | Sex (female/male) | BMI | OA site | Matched | Quality score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OA | Control | OA | Control | OA | Control | OA | Control | ||||||
| Toussirot-1, 2017 [8] | France | European | 23 | 22 | 64.5±0.2* | 59.7±0.1* | 23/0 | 22/0 | 29.9±0.9* | 24.9±0.7* | Knee | Sex | 7 |
| Toussirot-2, 2017 [8] | France | European | 27 | 28 | 64.5±0.2* | 59.7±0.1* | 0/27 | 0/28 | 29.9±0.9* | 24.9±0.7* | Knee | Sex | 7 |
| Tootsi, 2016 [9] | Estonia | European | 70 | 70 | 62.0±7.0 | 60.0±7.0 | 35/35 | 34/36 | 28.0±3.0 | 26.0±3.0 | Knee, hip | Age, sex | 7 |
| Cuzdan-1, 2015 [11] | Turkey | Turkish | 30 | 25 6 | 67.03±12.71 | 43.08±14.60 | 27/3 | 13/12 | 23.39±1.77 | 27.45±5.55 | Knee | NA | 6 |
| Cuzdan-2, 2015 [11] | Turkey | Turkish | 30 | 25 6 | 64.56±10.60 | 43.08±14.60 | 30/0 | 98/34 | 33.11±3.42 | 27.45±5.55 | Knee | NA | 6 |
| de Boer, 2012 [14] | Netherlands | European | 172 | 132 | 67.4±8.4 | 56.5±4.5 | 119/53 | 19/5 | 29.5±5.3 | 28.1±3.8 | Knee | Sex | 7 |
| Honsawek, 2010 [10] | Thailand | Asian | 76 | 24 | 69.8±1.1* | 71.2±1.5* | 62/14 | 26/0 | 26.1±0.6* | 25.5±0.6* | Knee, hip | Age, sex, BMI | 8 |
| Laurberg-1, 2009 [12] | Denmark | European | 22 | 26 | 63.0±10.3 | 46.0±14.4 | 22/0 | 0/19 | 29.9±5.2 | NA | Knee | Sex | 7 |
| Laurberg-2, 2009 [12] | Denmark | European | 13 | 19 | 65.0±7.8 | 54.0±7.4 | 0/13 | 20/0 | 27.1±4.0 | NA | Knee | Sex | 7 |
| Filkova-1, 2009 [13] | Czech | European | 27 | 20 | NA | NA | 27/0 | 20/0 | NA | NA | Knee, hip | Sex | 6 |
| Filkova-2, 2009 [13] | Czech | European | 48 | 20 | NA | NA | 48/0 | 20/0 | NA | NA | Knee, hip | Sex | 6 |
Table 2.
Meta-analysis of adiponectin levels in patients with OA compared to that in controls




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download