Abstract
Enthesitis-related arthritis (ERA) is a disease predominantly affecting the joints and entheses of the lower extremities and has the potential to eventually affect the sacroiliac joints and spine evolving to juvenile ankylosing spondylitis. ERA is also characterized by rheumatoid factor seronegativity, paucity of antinuclear antibody, and a strong association with the human leukocyte antigen-B27. ERA accounts for a higher proportion of juvenile idiopathic arthritis (JIA) cases in the Asian population compared to other populations. Advances in the understanding of ERA pathogenesis continue to progress and have led to the development of new treatments targeting pro-inflammatory cytokines. In particular, tumor necrosis factor-α inhibitors have become a main-stay of therapy for patients in whom therapy with anti-inflammatory drugs and/or disease-modifying anti-rheumatic drugs are inadequate or contraindicated. Compared to other JIA subtypes, ERA is associated with a poorer quality of life, worse function, and a higher likelihood of ongoing active disease after the initial treatment. Because the current guidelines for the management of ERA is not considered separately from other categories of JIA, there is a need for treatment guidelines specific to ERA to improve the overall disease outcomes.
Go to : 
REFERENCES
1. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004; 31:390–2.
2. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009; 68:777–83.

3. Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011; 70:25–31.

4. Boiu S, Marniga E, Bader-Meunier B, Mouy R, Compeyrot-Lacassagne S, Quartier P, et al. Functional status in severe juvenile idiopathic arthritis in the biologic treatment era: an assessment in a French paediatric rheumatology referral centre. Rheumatology (Oxford). 2012; 51:1285–92.

5. Demirkaya E, Ozen S, Bilginer Y, Ayaz NA, Makay BB, Unsal E, et al. The distribution of juvenile idiopathic arthritis in the eastern Mediterranean: results from the registry of the Turkish Paediatric Rheumatology Association. Clin Exp Rheumatol. 2011; 29:111–6.
6. Modesto C, Antón J, Rodriguez B, Bou R, Arnal C, Ros J, et al. Incidence and prevalence of juvenile idiopathic arthritis in Catalonia (Spain). Scand J Rheumatol. 2010; 39:472–9.

7. Solau-Gervais E, Robin C, Gambert C, Troller S, Danner S, Gombert B, et al. Prevalence and distribution of juvenile idiopathic arthritis in a region of Western France. Joint Bone Spine. 2010; 77:47–9.

8. Saurenmann RK, Rose JB, Tyrrell P, Feldman BM, Laxer RM, Schneider R, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007; 56:1974–84.

9. Weiss PF, Beukelman T, Schanberg LE, Kimura Y, Colbert RA. CARRA Registry Investigators. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol. 2012; 39:2341–51.

10. Yu HH, Chen PC, Wang LC, Lee JH, Lin YT, Yang YH, et al. Juvenile idiopathic arthritis-associated uveitis: a nationwide population-based study in Taiwan. PLoS ONE. 2013; 8:e70625.

11. Gmuca S, Xiao R, Brandon TG, Pagnini I, Wright TB, Beukelman T, et al. Multicenter inception cohort of en-thesitis-related arthritis: variation in disease characteristics and treatment approaches. Arthritis Res Ther. 2017; 19:84.

12. Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in en-thesitis-related arthritis. Arthritis Res Ther. 2014; 16:486.

13. Stoll ML, Patel AS, Punaro M, Dempsey-Robertson M. MR enterography to evaluate subclinical intestinal inflammation in children with spondyloarthritis. Pediatr Rheumatol Online J. 2012; 10:6.

14. Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990; 63:1099–112.
15. Breban M, Hammer RE, Richardson JA, Taurog JD. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993; 178:1607–16.

16. Geczy AF, Seager K, Bashir HV, de Vere-Tyndall A, Edmonds J. The role of Klebsiella in the pathogenesis of ankylosing spondylitis. II Evidence for a specific B27-associated marker on the lymphocytes of patients with ankylosing spondylitis. J Clin Lab Immunol. 1980; 3:23–8.
17. Cameron FH, Russell PJ, Easter JF, Wakefield D, March L. Failure of Klebsiella pneumoniae antibodies to cross-react with peripheral blood mononuclear cells from patients with ankylosing spondylitis. Arthritis Rheum. 1987; 30:300–5.
18. Rosenbaum JT, Davey MP. Time for a gut check: evidence for the hypothesis that HLA-B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum. 2011; 63:3195–8.

19. Stanevicha V, Eglite J, Zavadska D, Sochnevs A, Lazareva A, Guseinova D, et al. HLA B27 allele types in homogeneous groups of juvenile idiopathic arthritis patients in Latvia. Pediatr Rheumatol Online J. 2010; 8:26.

20. Srivastava R, Agnihotry S, Aggarwal R, Bajpai P, Aggarwal A. HLA-B27 subtypes in enthesitis-related arthritis category of juvenile idiopathic arthritis and ankylosing spondylitis in northern India. Clin Exp Rheumatol. 2015; 33:931–5.
21. Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybrid-ization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995; 38:499–505.

24. Chatzikyriakidou A, Voulgari PV, Drosos AA. What is the role of HLA-B27 in spondyloarthropathies? Autoimmun Rev. 2011; 10:464–8.

25. Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014; 57:44–51.

26. Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, et al. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002; 46:2972–82.

27. Bowness P, Ridley A, Shaw J, Chan AT, Wong-Baeza I, Fleming M, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011; 186:2672–80.

28. Kollnberger S, Bowness P. The role of B27 heavy chain dimer immune receptor interactions in spondyloarthritis. Adv Exp Med Biol. 2009; 649:277–85.

29. Hinks A, Martin P, Flynn E, Eyre S, Packham J. Subtype specific genetic associations for juvenile idiopathic arthritis: ERAP1 with the enthesitis related arthritis subtype and IL23R with juvenile psoriatic arthritis. Arthritis Res Ther. 2011; 13:R12.

30. Saxena N, Aggarwal A, Misra R. Elevated concentrations of monocyte derived cytokines in synovial fluid of children with enthesitis related arthritis and polyarticular types of juvenile idiopathic arthritis. J Rheumatol. 2005; 32:1349–53.
31. Mahendra A, Misra R, Aggarwal A. Th1 and Th17 predominance in the enthesitis-related arthritis form of juvenile idiopathic arthritis. J Rheumatol. 2009; 36:1730–6.

32. Gaur P, Misra R, Aggarwal A. Natural killer cell and gamma delta T cell alterations in enthesitis related arthritis category of juvenile idiopathic arthritis. Clin Immunol. 2015; 161:163–9.

34. Petty RE, Laxer RM, Lindsey CB, Wedderburn L. Textbook of pediatric rheumatology. 7th ed.Amsterdam: Elsevier;2016. p. 238–55.
35. Burgos-Vargas R, Vazquez-Mellado J. The early clinical recognition of juvenile-onset ankylosing spondylitis and its differentiation from juvenile rheumatoid arthritis. Arthritis Rheum. 1995; 38:835–44.

36. Flatø B, Hoffmann-Vold AM, Reiff A, Førre Ø, Lien G, Vinje O. Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum. 2006; 54:3573–82.

37. Sherry DD, Sapp LR. Enthesalgia in childhood: site-specific tenderness in healthy subjects and in patients with seronegative enthesopathic arthropathy. J Rheumatol. 2003; 30:1335–40.
38. Weiss PF. Diagnosis and treatment of enthesitis-related arthritis. Adolesc Health Med Ther. 2012; 2012; 67–74.

40. Oen K, Duffy CM, Tse SM, Ramsey S, Ellsworth J, Chédeville G, et al. Early outcomes and improvement of patients with juvenile idiopathic arthritis enrolled in a Canadian multicenter inception cohort. Arthritis Care Res (Hoboken). 2010; 62:527–36.

41. Rostom S, Amine B, Bensabbah R, Abouqal R, Hajjaj-Hassouni N. Hip involvement in juvenile idiopathic arthritis. Clin Rheumatol. 2008; 27:791–4.

42. Ramanathan A, Srinivasulu H, Colbert RA. Update on juvenile spondyloarthropathy. Rheum Dis Clin N Am. 2013; 39:767–88.
43. Alvarez-Madrid C, Merino R, De Inocencio J, García-Consuegra J. Tarsitis as an initial manifestation of juvenile spondyloarthropathy. Clin Exp Rheumatol. 2009; 27:691–4.
44. Minden K, Niewerth M, Listing J, Biedermann T, Bollow M, Schöntube M, et al. Long-term outcome in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002; 46:2392–401.

45. Chen HA, Chen CH, Liao HT, Lin YJ, Chen PC, Chen WS, et al. Clinical, functional, and radiographic differences among juvenile-onset, adult-onset, and late-onset ankylosing spondylitis. J Rheumatol. 2012; 39:1013–8.

46. Weiss PF, Xiao R, Biko DM, Chauvin NA. Sacroiliitis at diagnosis of juvenile spondyloarthritis assessed by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res (Hoboken). 2016; 68:187–94.
47. Stoll ML, Bhore R, Dempsey-Robertson M, Punaro M. Spondyloarthritis in a pediatric population: risk factors for sacroiliitis. J Rheumatol. 2010; 37:2402–8.

48. El Maghraoui A, Bensabbah R, Bahiri R, Bezza A, Guedira N, Hajjaj-Hassouni N. Cervical spine involvement in ankylosing spondylitis. Clin Rheumatol. 2003; 22:94–8.
49. Heiligenhaus A, Niewerth M, Ganser G, Heinz C, Minden K. German Uveitis in Childhood Study Group. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nationwide study in Germany: suggested modification of the current screening guidelines. Rheumatology (Oxford). 2007; 46:1015–9.

50. Stoll ML, Punaro M, Patel AS. Fecal calprotectin in children with the enthesitis-related arthritis subtype of juvenile idiopathic arthritis. J Rheumatol. 2011; 38:2274–5.

51. Weiss PF, Klink AJ, Behrens EM, Sherry DD, Finkel TH, Feudtner C, et al. Enthesitis in an inception cohort of en-thesitis-related arthritis. Arthritis Care Res (Hoboken). 2011; 63:1307–12.

52. Weiss PF, Colbert RA, Xiao R, Feudtner C, Beukelman T, DeWitt EM, et al. Development and retrospective validation of the juvenile spondyloarthritis disease activity index. Arthritis Care Res (Hoboken). 2014; 66:1775–82.

53. Schueller-Weidekamm C. [Inflammatory spinal disease: Spondyloarthritis: Importance of imaging]. Radiologe. 2015; 55:337–46. German.
54. Baek HJ, Shin KC, Lee YJ, Kang SW, Lee EB, Yoo CD, et al. Juvenile onset ankylosing spondylitis (JAS) has less severe spinal disease course than adult onset ankylosing spondylitis (AAS): clinical comparison between JAS and AAS in Korea. J Rheumatol. 2002; 29:1780–5.
55. Eder L, Barzilai M, Peled N, Gladman DD, Zisman D. The use of ultrasound for the assessment of enthesitis in patients with spondyloarthritis. Clin Radiol. 2013; 68:219–23.

56. Kim HK, Zbojniewicz AM, Merrow AC, Cheon JE, Kim IO, Emery KH. MR findings of synovial disease in children and young adults: Part 1. Pediatr Radiol. 2011; 41:495–511.

57. Jaganathan S, Goyal A, Gadodia A, Rastogi S, Mittal R, Gamanagatti S. Spectrum of synovial pathologies: a pictorial assay. Curr Probl Diagn Radiol. 2012; 41:30–42.

58. Mandl P, Navarro-Compán V, Terslev L, Aegerter P, van der Heijde D, D'Agostino MA, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. 2015; 74:1327–39.

59. Sung S, Kim HS, Kwon JW. MRI assessment of sacroiliitis for the diagnosis of axial spondyloarthropathy: comparison of fatsaturated T2, STIR and contrastenhanced sequences. Br J Radiol. 2017; 90:20170090.

60. Rudwaleit M, Jurik AG, Hermann KG, Landewé R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/ OMERACT MRI group. Ann Rheum Dis. 2009; 68:1520–7.
61. Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009; 68(Suppl 2):ii1–44.

62. Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res (Hoboken). 2011; 63:465–82.

63. Gmuca S, Weiss PF. Evaluation and Treatment of Childhood Enthesitis-Related Arthritis. Curr Treatm Opt Rheumatol. 2015; 1:350–64.

64. Huppertz HI, Tschammler A, Horwitz AE, Schwab KO. Intraarticular corticosteroids for chronic arthritis in children: efficacy and effects on cartilage and growth. J Pediatr. 1995; 127:317–21.

65. Allen RC, Gross KR, Laxer RM, Malleson PN, Beauchamp RD, Petty RE. Intraarticular triamcinolone hexacetonide in the management of chronic arthritis in children. Arthritis Rheum. 1986; 29:997–1001.

66. van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017; 76:978–91.
69. van Rossum MA, Fiselier TJ, Franssen MJ, Zwinderman AH, ten Cate R, van Suijlekom-Smit LW, et al. Sulfasalazine in the treatment of juvenile chronic arthritis: a randomized, double-blind, placebo-controlled, multicenter study. Dutch Juvenile Chronic Arthritis Study Group. Arthritis Rheum. 1998; 41:808–16.
70. Burgos-Vargas R, Vázquez-Mellado J, Pacheco-Tena C, Hernández-Garduño A, Goycochea-Robles MV. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Ann Rheum Dis. 2002; 61:941–2.

71. Henrickson M, Reiff A. Prolonged efficacy of etanercept in refractory enthesitis-related arthritis. J Rheumatol. 2004; 31:2055–61.
72. Horneff G, De Bock F, Foeldvari I, Girschick HJ, Michels H, Moebius D, et al. Safety and efficacy of combination of etanercept and methotrexate compared to treatment with etanercept only in patients with juvenile idiopathic arthritis (JIA): preliminary data from the German JIA Registry. Ann Rheum Dis. 2009; 68:519–25.

73. Otten MH, Prince FH, Twilt M, Ten Cate R, Armbrust W, Hoppenreijs EP, et al. Tumor necrosis factor-blocking agents for children with enthesitis-related arthritis–data from the dutch arthritis and biologicals in children register, 1999-2010. J Rheumatol. 2011; 38:2258–63.
74. Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, et al. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther. 2012; 14:R230.

75. Yates M, Hamilton LE, Elender F, Dean L, Doll H, MacGregor AJ, et al. Is Etanercept 25 mg once weekly as effective as 50 mg at maintaining response in patients with ankylosing spondylitis? A randomized control trial. J Rheumatol. 2015; 42:1177–85.

76. Cantini F, Niccoli L, Cassarà E, Kaloudi O, Nannini C. Duration of remission after halving of the etanercept dose in patients with ankylosing spondylitis: a randomized, prospective, long-term, follow-up study. Biologics. 2013; 7:1–6.

77. Song IH, Althoff CE, Haibel H, Hermann KG, Poddubnyy D, Listing J, et al. Frequency and duration of drug-free remission after 1 year of treatment with etanercept versus sulfasalazine in early axial spondyloarthritis: 2 year data of the ESTHER trial. Ann Rheum Dis. 2012; 71:1212–5.

78. Haibel H, Heldmann F, Braun J, Listing J, Kupper H, Sieper J. Long-term efficacy of adalimumab after drug withdrawal and retreatment in patients with active non-radiographically evident axial spondyloarthritis who experience a flare. Arthritis Rheum. 2013; 65:2211–3.

79. Flatø B, Aasland A, Vinje O, Forre O. Outcome and predictive factors in juvenile rheumatoid arthritis and juvenile spondyloarthropathy. J Rheumatol. 1998; 25:366–75.
80. Selvaag AM, Lien G, Sørskaar D, Vinje O, Førre Ø, Flatø B. Early disease course and predictors of disability in juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3 year prospective study. J Rheumatol. 2005; 32:1122–30.
Go to : 
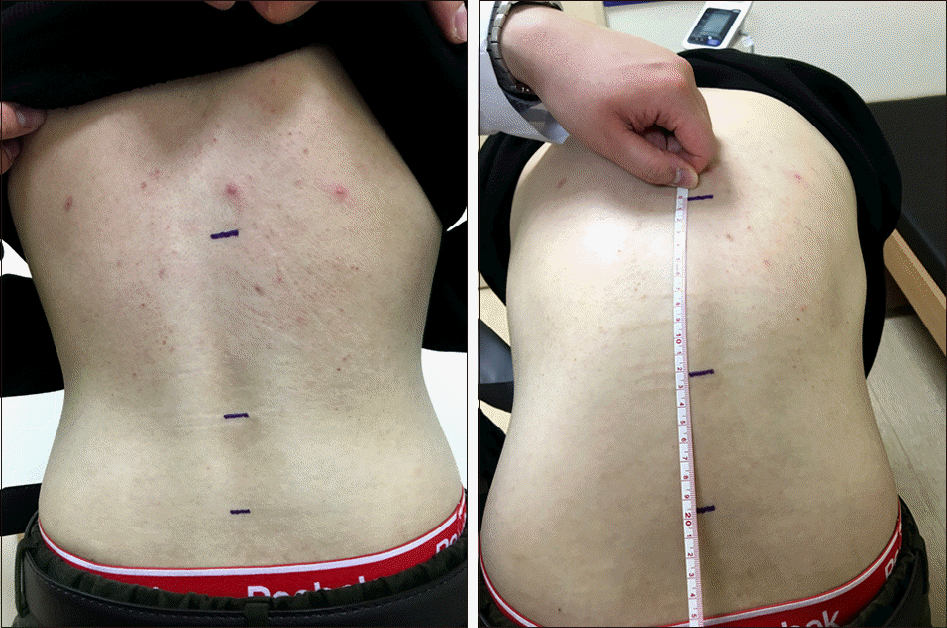 | Figure 1.Modified Schober's test. With patient standing up-right, two points are marked on the back 5 cm below and 10 cm above the horizontal line joining the dimples of Venus which is used as a mark for the lumbosacral junction. On maximal forward flexion, keeping the knees straight, an increase of the distance between the top and bottom points reflects lumbosacral spinal mobility and is considered normal if more than 5 cm. |
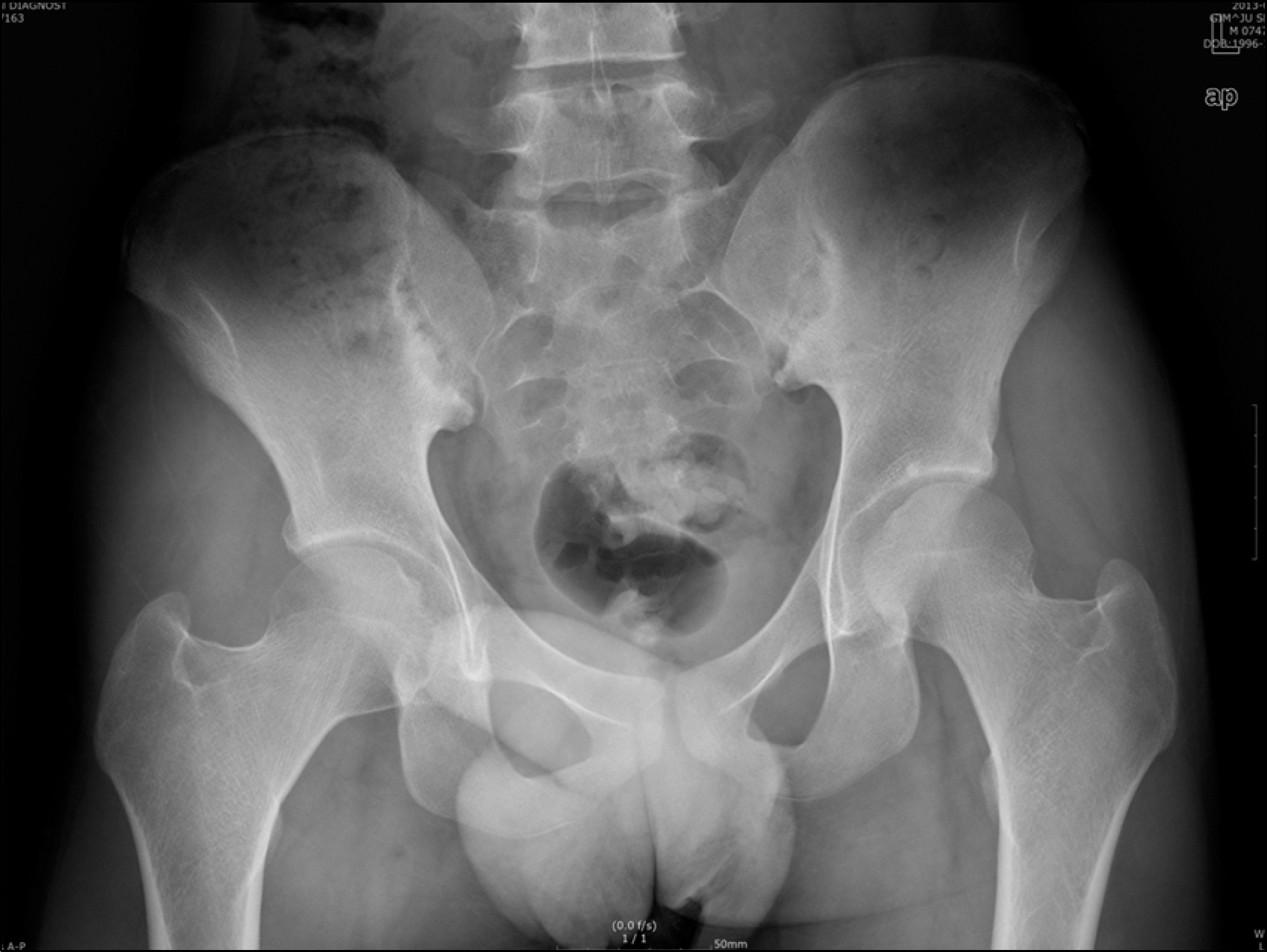 | Figure 2.Anteroposterior view of the pelvis shows joint space narrowing, bone erosions, and reactive sclerosis in the sacroiliac joints. |
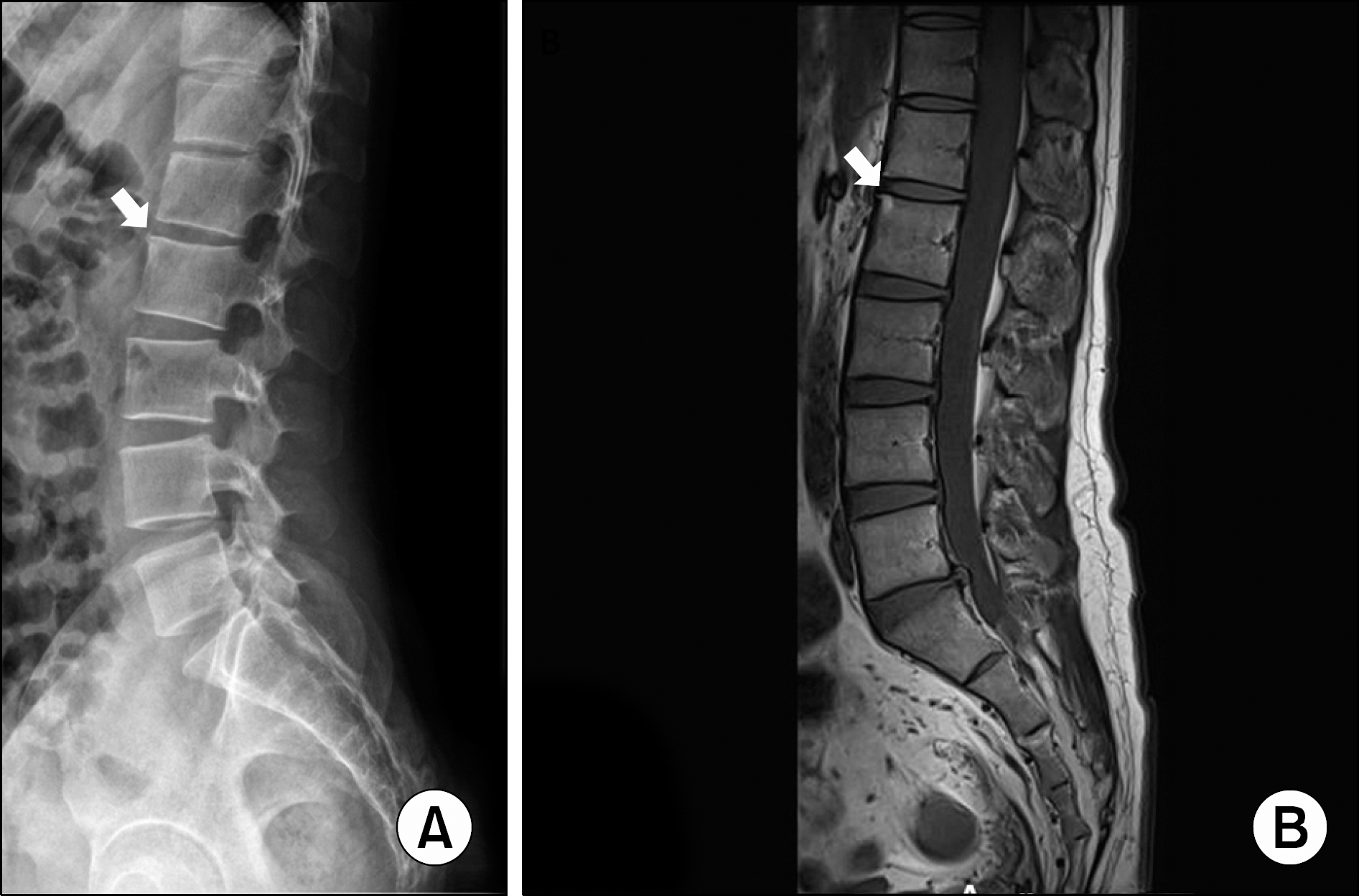 | Figure 3.Corner lesion in 15 year old male with en-thesitis-related arthritis. Plain radiograph (A) and T1-weighted magnetic resonance imaging (B) of spine show increased signal at the anterior aspect of the superior endplate of L2 (arrows). |
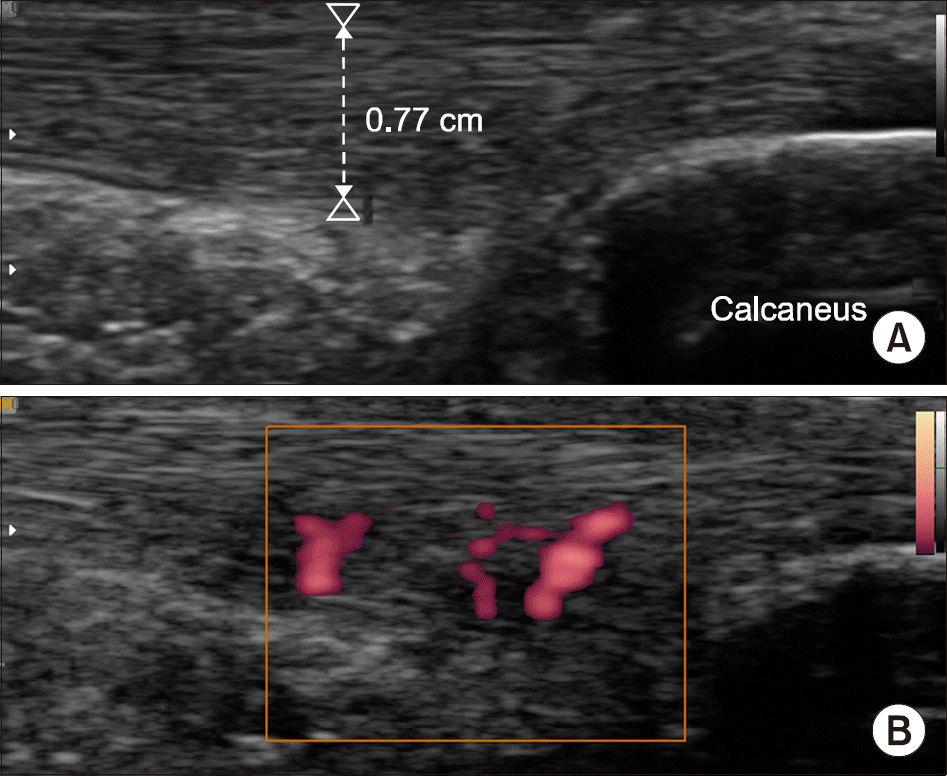 | Figure 4.Achilles tendon enthesitis in a 14 year old boy. (A) Longitudinal grayscale ultrasound image demonstrates increased thickness of Achilles tendon (arrowheads) with hypo-echogenicity and loss of fibrillar pattern in deep layer. (B) Power Doppler images demonstrates increased signals within the tendon consistent with hyperemia. |
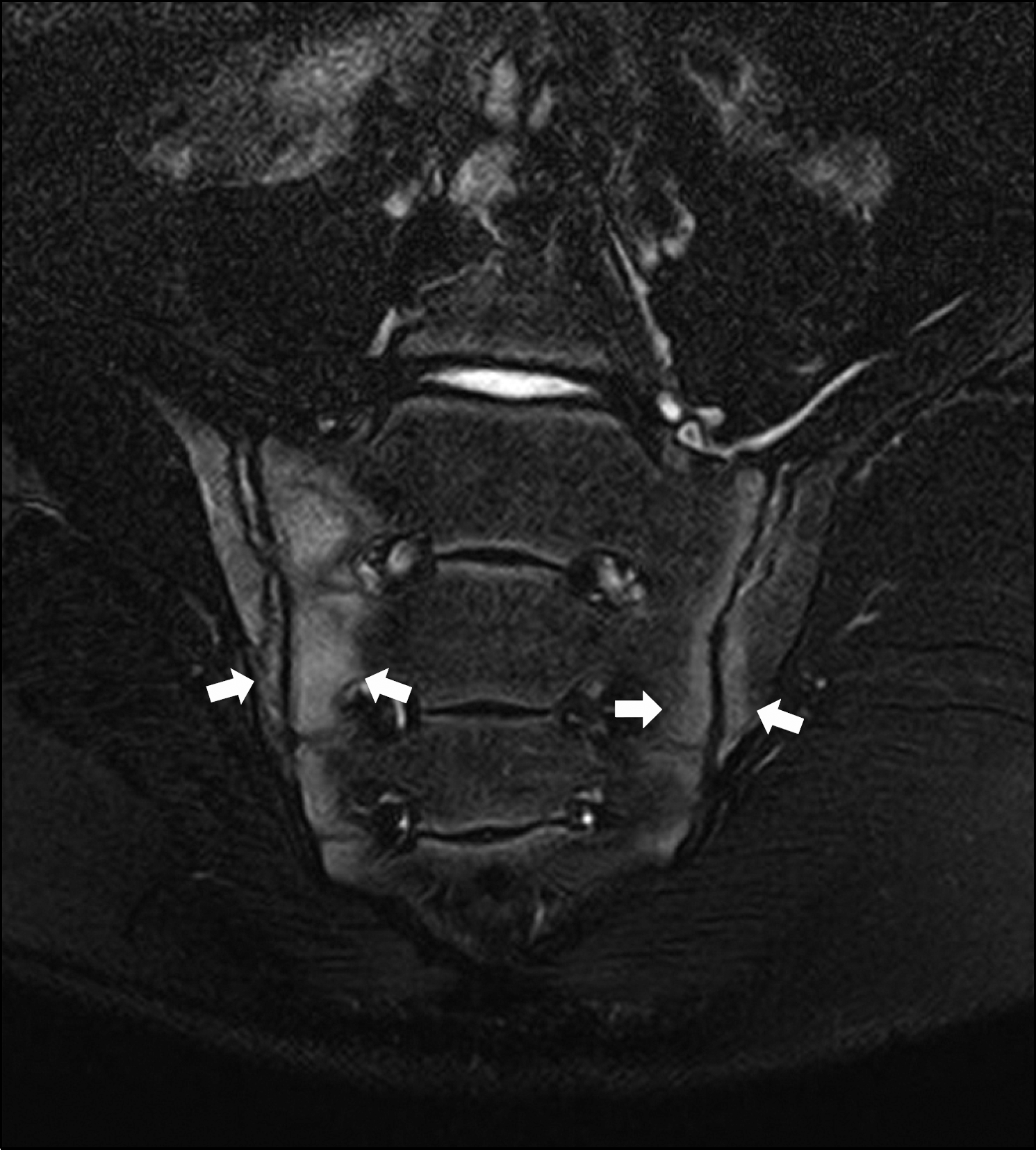 | Figure 5.Coronal T1-weighted magnetic resonance imaging of pelvis in 15 year old male with enthesitis-related arthritis. There is active sacroiliitis with bone marrow edema (arrows) on both iliac and sacral sides of the sacroiliac joints. |
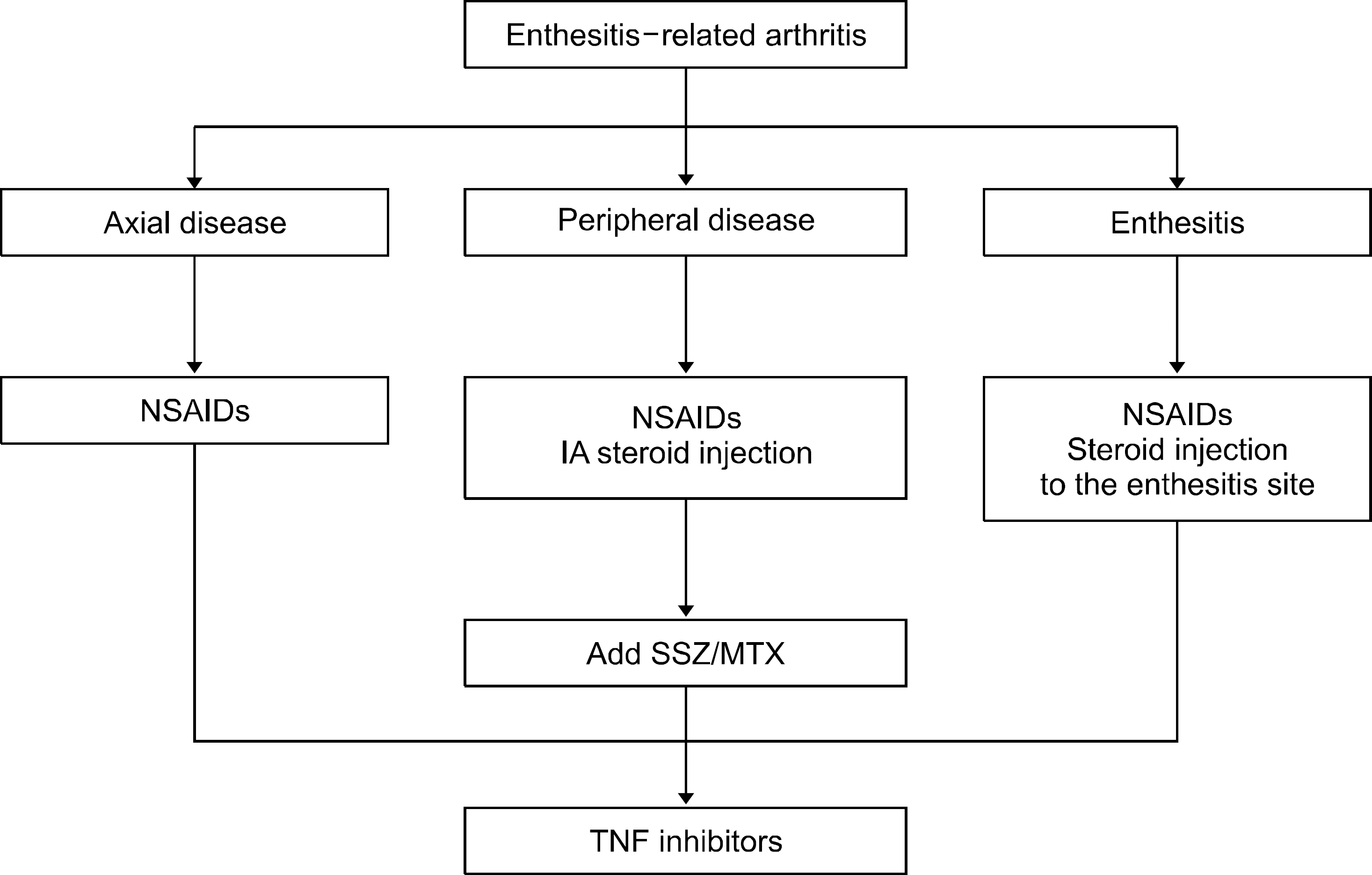 | Figure 6.Flow of treatments for patients with enthesitis-related arthritis. NSAIDs: non-steroidal anti-inflammatory drugs, IA: intraarticular, SSZ: sulfasalazine, MTX: methotrexate, TNF: tumor necrosis factor. |
Table 1.
International League of Associations for Rheumatology (ILAR) classification criteria for enthesitis-related arthritis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download