Abstract
Metronidazole is an antimicrobial agent widely used for the treatment of anaerobic infection or antibiotics-associated diarrhea. It is generally thought to be safe, but can induce reversible toxic encephalopathy in the case of excessive or cumulative over-dose. Metronidazole-induced encephalopathy generally demonstrates the characteristic features of typical lesion location and bilaterality on magnetic resonance imaging (MRI). We report a case of metronidazole-induced encephalopathy with the involvement of asymmetric white matter. To our knowledge, only a few cases have been reported with respect to white matter lesion characteristics on MRI with diffusion-weighted images.
The brain is susceptible to toxicity associated with various drugs and chemical agents. Along with heavy alcohol abuse, which results in chronic or acute brain damage, innumerable other drugs have been reported to induce toxic encephalopathy. Imaging workup plays an important role in the diagnosis of most toxic diseases. Those diseases usually appear in a symmetric fashion, especially with the involvement of the deep gray nuclei and cerebral cortex. Radiologists should be able to recognize and suggest a potential diagnosis of toxic encephalopathy, since early withdrawal of the chemical agents halts the progression of neuropathy and improves clinical prognosis.
Metronidazole-induced encephalopathy is a rare form of toxic encephalopathy associated with the use of metronidazole (1) and typically shows symmetric involvement of the dentate nucleus of the cerebellum (2). Metronidazole is one of the most widely-used treatments for anaerobic infection, Crohn's disease, antibiotics-associated diarrhea, or clostridium difficile-associated disease (2,3). Due to its relatively low cost and availability in either oral or intravenous forms, as well as its rapid antibacterial effectiveness, metronidazole is considered to be the gold standard antibiotic agent with anaerobic activity. Metronidazole also has good cellular penetration, as well as cerebrospinal fluid and central nervous system penetration, thereby having notable effectiveness in the treatment of anaerobic brain abscess (2). It is generally safe at appropriate dosages, but it can induce neurologic complications, especially when the dose exceeds 2 g/day, or when a cumulative dose of 21–135 g has been used (1,2). The clinical symptoms consist of variable forms of neurologic deficit such as ataxia, encephalopathy, or peripheral neuropathy (4).
Herein, we report a patient with metronidazole-induced encephalopathy with full recovery after cessation of drug and review the imaging characteristics in an atypical lesion location on magnetic resonance imaging (MRI).
A 58-year-old woman who was admitted to our institution for rehabilitative care after spinal surgery for her central canal stenosis presented with abrupt right-sided hemiplegia and aphasia. At the time when her neurologic symptoms developed, her vital signs were stable and her laboratory results were within normal ranges. With suspicion of acute stroke based on neurologic examination, brain MRI (Verio: Siemens, Erlangen, Germany) was performed. Diffusion-weighted imaging (DWI) showed multifocal high signal intensities (SI) at the bilateral corpus callosum and the left cerebral subcortical white matter (WM) (Fig. 1). The subcortical WM in the left frontoparietal lobe and the genu of the corpus callosum showed low values on the apparent diffusion coefficient (ADC) map, representing cytotoxic edema. The splenium of corpus callosum, however, increased the ADC value and increased SI on T2 fluid attenuation inversion recovery (FLAIR) images, representing vasogenic edema. Gadolinium-enhanced study and MR angiography were negative. Notably, she had been treated with oral metronidazole (1.5 g/day) for about three months for the treatment of chronic diarrhea due to clostridium difficile-associated disease. Considering the patient's medical history and bilateral corpus callosum involvement on MRI, we suggested metronidazole-induced encephalopathy as the most likely diagnosis. Upon cessation of metronidazole, her neurologic symptoms gradually regressed and she underwent follow-up MRI after a week (Fig. 2). The previous lesions were markedly resolved although mild high SI at the splenium of the corpus callosum remained on DWI and T2 FLAIR images. On repeated follow-up MRI after a month (Fig. 3), near-complete resolution of the previous lesions was observed along with improvement of her neurologic symptoms.
The precise mechanism of metronidazole-induced toxicity has not yet been clarified. Previous reports have suggested following the mechanisms of metronidazole neurotoxicity; metronidazole's intermediate metabolites modulate inhibitory neurotransmitter GABA receptor, and the reactions with catecholamine neurotransmitter generate semiquinone and nitro anion neurotoxic radicals (1). According to prior animal studies focused on histologic findings, the spongiform change within neurons as well as the degeneration and selective loss of Purkinje cells were detected in the cerebellum, vestibular system, and medulla oblongata (5,6,7,8). Yet, irreversible histologic change, such as demyelination or necrosis, could not clearly explain the reversibility of imaging or clinical features as described in the study of Ahmed et al. (9). We assume that the transient axonal swelling is one of the possible reasons for this.
Previous studies have suggested that the ADC values depend on lesion location. In a study by Kim et al. (2), the lesion in the splenium showed a decreased ADC value and cytotoxic edema. On the contrary, in our study, the lesion in the same location showed an increased ADC value and vasogenic edema. Therefore, further studies regarding the variability of ADC values in metronidazole-induced encephalopathy are needed.
Kim et al. (2) also reported the lesion distribution of metronidazole-induced encephalopathy based on 20 patients of their case studies as well as previously-reported data: cerebellar dentate nuclei (n = 17, 85%), the midbrain (n = 11, 55%), corpus callosum (n =10, 50%), pons (n = 7, 35%), medulla (n = 6, 30%), subcortical WM of the hemisphere (n = 5, 25%) and basal ganglia (n = 4, 20%). They concluded that bilateral and symmetric involvement of the cerebellar dentate nuclei, dorsal medulla, dorsal pons, midbrain, and splenium of the corpus callosum was a very characteristic feature of metronidazole-induced encephalopathy.
In our patient, among the structures most commonly affected, only the splenium showed abnormality. Furthermore, the asymmetric signal change in the subcortical WM of the left cerebral hemisphere was considered to be an unusual feature of metronidazole-induced encephalopathy or other metabolic disorder. Therefore, we initially suggested acute infarction or demyelinating disorder as a differential diagnosis, even though the possibility of these seemed low. Considering the patient's history of metronidazole administration, the bilateral symmetric involvement of the corpus callosum on initial MRI, and their complete recovery on follow-up MRI a month after cessation of metronidazole, we made a confirmative diagnosis of drug-induced encephalopathy.
In regard to the possible mechanism of the asymmetric WM involvement, the precise etiology is unknown to the best of our knowledge, and more research is needed.
Metronidazole-induced encephalopathy presents as bilateral and symmetric involvement of the dentate nucleus in the cerebellum, midbrain, pons, and splenium of the corpus callosum in most previously-reported cases, but unusual manifestations, such as our case, should be noted. In spite of the unusual manifestations, we could make a confirmative diagnosis based on the clinical history and reversibility on follow-up MR images. Radiologists should be aware of this and suggest the diagnosis of metronidazole-induced encephalopathy in patients with a history of metronidazole administration, because a full recovery can be expected upon drug withdrawal.
Figures and Tables
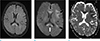 | Fig. 1Initial MRI of the brain of a 58-year-old woman (a–c). Diffusion-weighted imaging (b) showed multifocal high signal intensities at the genu and splenium of the bilateral corpus callosum and subcortical white matter of the left cerebral hemisphere (black and white arrows). The splenium of the corpus callosum (white arrow) showed no increased value on the apparent diffusion coefficient (ADC) map (c) and high signal intensity on a T2 fluid attenuation inversion recovery (FLAIR) image (a). The others showed a decreased value on the ADC map and isointensity on a T2 FLAIR image. |
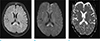 | Fig. 2One-week follow-up MRI of the brain after discontinuation of metronidazole (a–c). The previous lesions are markedly resolved on diffusion-weighted imaging (DWI) (b) and apparent diffusion coefficient (ADC) map (c), although the lesion in the splenium of the corpus callosum remained on a T2 fluid attenuation inversion recovery (FLAIR) image (a) and DWI. |
References
1. Bahn Y, Kim E, Park C, Park HC. Metronidazole induced encephalopathy in a patient with brain abscess. J Korean Neurosurg Soc. 2010; 48:301–304.

2. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007; 28:1652–1658.

3. Park HS, Han DS. Management of antibiotics-associated diarrhea. Korean J Gastroenterol. 2009; 54:5–12.

4. Graves TD, Condon M, Loucaidou M, Perry RJ. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci. 2009; 285:238–240.

5. Dow SW, LeCouteur RA, Poss ML, Beadleston D. Central nervous system toxicosis associated with metronidazole treatment of dogs: five cases (1984–1987). J Am Vet Med Assoc. 1989; 195:365–368.
6. Olson EJ, Morales SC, McVey AS, Hayden DW. Putative metronidazole neurotoxicosis in a cat. Vet Pathol. 2005; 42:665–669.

7. Scharer K. Selective alterations of Purkinje cells in the dog after oral administration of high doses of nitroimidazole derivatives (author's transl). Verh Dtsch Ges Pathol. 1972; 56:407–410.
8. von Rogulja P, Kovac W, Schmid H. Metronidazol encephalopathy in rats. Acta Neuropathol. 1973; 25:36–45.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download