Abstract
Sweet's syndrome also known as acute neutrophilic dermatosis is a multisystem inflammatory disorder characterized by fever, malaise, leukocytosis, and skin lesions. Sweet's syndrome affects multiple organs though only rarely does it affect the central nervous system (CNS) when it does it is called Neuro-Sweet disease (NSD). We report on a case study of a biopsy-proven NSD in a 50 year old man. Serial magnetic resonance imaging (MRI) showed repeated CNS involvement of Sweet's syndrome after a respiratory tract infection preceded it. On the MRI, T2 hyperintense lesions occurred at multiple sites and disappeared after steroid therapy.
Sweet's syndrome also referred to as acute neutrophilic dermatosis was first described by Robert Douglas Sweet in 1964 (1). This unusual inflammatory disorder is characterized by fever, malaise, leukocytosis, and distinctive skin lesions. Painful erythematous plaques usually affect the face, neck, upper body, and limbs. Skin biopsies show dense dermal infiltration of neutrophils without any histological signs of vasculitis (2). Sweet's syndrome is known to involve multiple organs though central nervous system (CNS) involvement is rare. When the CNS is involved, it is called Neuro-Sweet disease (NSD). NSD is considered a distinct entity and separate diagnostic criteria was proposed and adopted that accounts for idiopathic encephalitis and meningitis (3). Here we report on a case of relapsing NSD associated with a prior upper respiratory infection (URI), discuss indicators in the imaging results, and briefly review the literature.
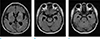
In January 2018, a 50-year-old male patient was admitted to our hospital for fever and confused mental condition. Fever, skin rash, sore throat, and myalgia persisted for one week before admission. A few days before admission, acute tonsillitis was diagnosed at another hospital and antibiotics were prescribed. On physical examination, painful erythematous nodules were observed on the face, neck, upper body, and limbs. It spread gradually from the face to limbs (Fig. 1). The patient also had oral ulcers that he reported had appeared three days earlier. In addition, he had a high fever (39℃) and was confused and disoriented when he arrived. Other neurologic exams were normal though laboratory testing revealed WBC (17.72 × 103/µl), neutrophil count (12.53 × 103/µl), ESR (56 mm/hr) and CRP (11.10 mg/dL) were elevated. Antiviral and immunological tests were negative. Magnetic resonance imaging (MRI) fluid-attenuated inversion recovery (FLAIR) images revealed multiple hyperintense lesions in the left frontal lobe, left temporal lobe, left thalamus, pons, and midbrain that showed no definite diffusion restriction on diffusion weighted imaging (DWI) and no augmentation on post-contrast images (Fig. 2).
About 10 years prior, the patient was diagnosed with viral meningitis at another hospital. At the time symptoms improved after supportive care. About two years later in July 2010, transient memory loss occurred after an upper respiratory tract infection that lasted for several months. He also experienced symptoms like those present at the time at this admission. In May 2016, erythematous nodules affected his entire body for two to three weeks. These skin lesions first appeared on his face and then spread to his body. Cough and mucus was also reported, and he was treated for one week under the diagnosis of pneumonia at the other hospital. He visited the emergency room of a local university hospital with complaints of disorientation and confused mental state, at the time, laboratory tests showed WBC count (17.72 × 103/µl), ESR (60 mm/hr) and CRP (14 mg/dL) were elevated. Cerebrospinal fluid (CSF) examination revealed a protein elevation (201 mg/dL). MR FLAIR imaging showed multiple lesions with abnormally increased signal intensity in the left frontal lobe, both temporal lobes, both basal ganglia, and insula that showed no definite diffusion restrictions on DWI and no development on post-contrast images (Fig. 3). Skin biopsies showed neutrophil infiltration on the dermis. Based on considerations of all clinical and imaging findings, NSD involving the central nervous system was presumptively diagnosed. Steroids were started and after treatment for 10 days, postural disability showed improvement and skin lesions disappeared. Follow up FLAIR images revealed the extent of multiple hyperintense lesions in the whole brain had diminished (Fig. 4).
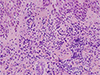
In view of the patient's medical history, we suspected NSD recurrence and performed a skin biopsy for confirmation. Histological examination of skin lesions revealed neutrophilic infiltration and as a result we diagnosed recurrence of NSD (Fig. 5). He responded well after five days of steroid therapy and almost recovered completely. Follow up FLAIR images taken one month after steroid therapy cessation demonstrated multiple abnormal high signals across the entire brain had disappeared (Fig. 6).
Sweet's syndrome is a multisystem inflammatory disorder characterized by acute neutrophil dermatosis (1). It may be associated with upper respiratory infection, pregnancy, inflammatory bowel disease, malignant disease, or drugs (4). Sweet's syndrome involves multiple organs including eyes, lungs, liver, kidneys, bone marrow, muscles, and the gastrointestinal tract. CNS involvement is rare and is believed to occur due to aseptic neutrophilic infiltration resulting in meningoencephalitis (5).
NSD first proposed as a distinct entity in 1999 is defined as the co-occurrence of encephalitis or meningitis and the classic skin lesions of Sweet's syndrome (6). NSD occurs more frequently in men with a male to female ratio of 1.3 to 1. In Asians it is more common in those aged 30 to 60 years (5).
The most common neurologic symptoms of NSD include headache, convulsions, and consciousness disturbances (7). A fever of over 38℃ is typically observed with NSD. CSF studies usually demonstrate elevated protein and pleocytosis, with a predominance of lymphocytes. Laboratory tests reveal neutrophil, CRP and ESR elevations (5). The pathogenesis of NSD remains unknown, although it is believed to be a manifestation of increased neutrophil and cytokine chemotaxis because of infection or malignancy. NSD responds well to systemic corticosteroid therapy (8). Clinical criteria for the diagnosis of NSD was published in 2005 and was based on neurological, dermatological, other features and HLA associations (3). For diagnosing NSD these factors are important: 1) good responsiveness to systemic glucocorticoid, 2) neutrophilic infiltration without vasculitis in skin lesions (erythematous plaques of nodules), and 3) the presence of HLA-B54 and/or HLA-Cw1 (3). According to the criteria, our case was classified as probable NSD. The involvement of the central nervous system of NSD is usually transient though it is known to recur in some cases (6). NSD can reportedly recur during steroid abatement (3).
The sites most affected in NSD are the basal ganglia, thalamus, and brainstem (7) though lesions may occur without predilection in any region of the central nervous system (9). In CT scans, lesions are hypodense and usually unremarkable. The MRIs T2-weighted or FLAIR images show hyperintense lesions in the basal ganglia, thalamus, hippocampus, subcortical regions, and brainstem. Some lesions show enhancement though some do not (3). Lesions that showed restricted diffusion on the DWI were reported for the diagnosis though no restricted diffusion was observed in our patient (7). As in most cases including ours steroid therapy resolved abnormal findings on the MRIs (3).
NSD shares clinical features and imaging results with Neuro-Behcet's disease (NBD). Our patient had an oral ulcer that suddenly developed three days before admission. He did not develop genital ulcers or ophthalmic involvement. A skin biopsy demonstrated erythema without definite vasculitis. NSD and NBD may both exhibit hyperintensive sites on MR T2-weighted and FLAIR images. NBD has a predilection for being located on the basal ganglia and brain stem, whereas NSD affects multiple sites without predilection in the central nervous system. Furthermore, acute active lesions of NBD often show augmentation, whereas NSD lesions do not. In addition, NBD lesions often involve the ventral side of the brain stem, whereas those of NSD are usually located in the dorsal side (10). In our patient, multiple nonaugmented lesions were observed at variable sites of the central nervous system without a pattern. In addition, CNS involvement in NSD is usually transient, whereas NBD commonly follows a progressive course. A series of MRI scans in our patient showed that encephalitis occurred about four times over a 10 year period. Recurrent encephalitis and the skin lesions were benign. And the patient recovered from each episode without complication. Other differential diagnoses of NSD include viral encephalitis and demyelinating diseases such as multiple sclerosis. Viral encephalitis and multiple sclerosis may also show multiple T2 hyperintensities in the brain. Therefore, it is difficult to differentiate from NSD only through imaging findings. The culture of the CSF sample from our patient was negative for bacteria, fungi, and antibodies against viruses. Demyelinating diseases, such as multiple sclerosis, were also excluded because of the acute onset of symptoms and rapid recovery. And the MRI did not show characteristic features of multiple sclerosis like juxtacortical and periventricular white matter hyperintensities (7).
The association between Sweet's syndrome and URI infection has been previously described in the literature (4). However, in our case NSD appeared to be associated with an upper respiratory tract infection. These relationships may be due to factors associated with inflammatory reaction from the preceding infection. When a patient has neurologic symptoms with skin lesions and has a preceding respiratory infection, NSD should be considered in the differential diagnosis. And if NSD is suspected, it is important that early skin biopsy be performed for diagnosis. Awareness of the clinical and imaging findings of NSD is important for avoiding unnecessary empirical therapy and to initiate rapid steroid therapy.
Figures and Tables
 | Fig. 1Clinical photograph of the patient showing multiple red erythematous plaques on the face and neck. |
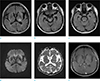 | Fig. 2Axial FLAIR MR images (a–c) revealed multiple hyperintense lesions in the left frontal lobe, left temporal lobe, left thalamus, pons and midbrain that showed no definite diffusion restriction on DWI (d, e) and no enhancement on contrast-enhanced T1WI (f). |
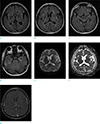 | Fig. 3Axial FLAIR images performed 2 years before this presentation (a–d) demonstrated multiple high signal intensity lesions in the left frontal lobe, both temporal lobes, both basal ganglia and insula. The lesions did not show diffusion restriction on DWI (e, f) and contrast enhancement T1WI (g). |
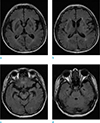 | Fig. 4Follow up FLAIR images taken after steroid therapy (a–d) showing the extent of multiple hyperintense lesions that diminished in the whole brain. |
References
1. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964; 76:349–356.
2. Cohen PR, Kurzrock R. Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol. 2003; 42:761–778.

3. Hisanaga K, Iwasaki Y, Itoyama Y. Neuro-Sweet Disease Study Group. Neuro-Sweet disease: clinical manifestations and criteria for diagnosis. Neurology. 2005; 64:1756–1761.

4. Cohen PR. Sweet's syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007; 2:34.

5. Hiari N, Borland C. A 47-year-old man with neuro-Sweet syndrome in association with Crohn's disease: a case report. J Med Case Rep. 2009; 3:8997.

6. Hisanaga K, Hosokawa M, Sato N, Mochizuki H, Itoyama Y, Iwasaki Y. “Neuro-sweet disease”: benign recurrent encephalitis with neutrophilic dermatosis. Arch Neurol. 1999; 56:1010–1013.
7. Singh JS, Costello F, Nadeau J, Witt S, Trotter MJ, Goyal M. Case 176: Neuro-sweet syndrome. Radiology. 2011; 261:989–993.

8. Kim YK, Bang D. Sweet's syndrome associated with bacterial meningitis. Ann Dermatol. 1996; 8:66–69.

9. Akiba C, Esaki T, Ando M, et al. Possible neuro-Sweet disease mimicking brain tumor in the medulla oblongata--case report. Neurol Med Chir (Tokyo). 2011; 51:140–114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download