Abstract
In contrast to well-known imaging findings of intracranial epidermoid cysts on magnetic resonance imaging, those of intracranial squamous cell carcinoma (SqCC) are relatively unknown. We present a case of coexistence of intracranial SqCC and epidermoid cyst, with consecutive follow up over 14 months. Based on our case, a solid enhancing portion adjacent to a typically-looking epidermoid cyst may become a clue for coexistence of intracranial SqCC. An initial contrast enhancement and/or heterogeneous signal on diffusion weighted imaging may become a useful diagnostic clue, but more importantly, sudden rapid growth is important in formulating diagnosis.
Intracranial squamous cell carcinoma (SqCC) is a rare condition. Intracranial epidermoid cysts, on the other hand, are more common, slowly growing indolent tumors that they constitute 1% of all intracranial tumors (1). One hypothesis for development of intracranial SqCC is from malignant transformation of intracranial epidermoid cyst (2) and there have been up to 44 such case reports since first reported by Ernst et al. in 1912. In contrast to well-known imaging findings of intracranial epidermoid cysts on magnetic resonance imaging (MRI) that are characterized by low signal intensity on T1-weighted image (T1WI) and high signal intensity on T2-weighted image (T2WI) (3), imaging findings of intracranial SqCC are relatively unknown. Also, there has been no report demonstrating serial imaging findings of coexistence of an intracranial SqCC and epidermoid cyst. Here, we report a case of coexistence of both intracranial SqCC and epidermoid cyst, and discuss possible mechanisms of coexistence based on the literature review.
A 71-year-old male presented with a 1-year history of visual disturbance in the right eye. He also complained of abnormal sensation on the right side of the face in the territory of the right trigeminal nerve. The symptoms gradually progressed for 2 months despite medical management including analgesics. He visited a local hospital and MRI was performed.
Fluid-attenuated inversion recovery (FLAIR) image revealed a 2.6 cm, lobulated and non-enhancing lesion in the basal cistern (Fig. 1a, b) with homogeneous diffusion restriction (Fig. 1c, d). Another 1.3 cm, enhancing lesion in the right cerebellopontine (CP) angle in the contrast-enhanced T1WI (Fig. 1e) was also noted. In diffusion-weighted image (DWI), the enhancing lesion (in the right cerebellopontine angle) showed heterogeneous diffusion restriction, which was different from the lesion in the basal cistern (Fig. 1f, g). At that time, the right CP angle lesion was diagnosed as inflammatory change associated with the basal cistern lesion, however when it is reviewed, it is difficult to rule out the possibility of intracranial SqCC. Cerebrospinal-fluid analysis was negative for malignant cells. After the diagnosis, he was followed up regularly and managed with dexamethasone and analgesics. The symptoms got worse, however, and the ophthalmologist decided to admit the patient to our hospital.
On the second visit, ophthalmologic examination revealed impaired ophthalmic nerve division (V1) with corneal ulceration on the right side (Fig. 2). The second MRI exam was taken 5 months after the first exam, and the enhancing lesion in the right CP angle slightly increased in its size by 2.1 cm when measured in the axial contrast-enhanced T1WI (Fig. 3). The ophthalmologist diagnosed his corneal ulceration as neurotropic keratitis, which is caused by damage of the V1 nerve branch.
On the third visit, the neurotropic keratitis progressed despite proper treatment and neurological examination revealed hypoesthesia in the territory of the maxillary nerve division (V2) and mandibular nerve division (V3). The third MRI was taken 9 months after the second MRI (14 months later from the first exam). The third MRI revealed that the enhancing lesion in the right CP angle further increased in its size by 3.4 cm when measured in the axial contrast-enhanced T1WI. FLAIR image also revealed high signal intensity in the adjacent right middle cerebellar peduncle due to peritumoral edema (Fig. 4). Based on these imaging findings with interval growth and resistance to anti-inflammatory treatment, the enhancing lesion was considered to have malignant tumor such as SqCC. On the other hand, the non-enhancing lesion in the basal cistern did not show any change in its size or signal intensity over time. His physician decided to admit the patient to our hospital and planned for resection of these lesions.
Surgery was performed through the right retro-mastoid suboccipital craniotomy. The overlying dura seemed to be normal. The tumor was located anterior to the 7th and 8th cranial nerve complex, displacing the 5th cranial nerve superiorly. Although the tumor was adherent severely to the adjacent neurovascular structure, most of the tumor was removed after careful dissection except some portion within the Meckel's cave due to dense adherence and locational limitation. There was no record of rupture of epidermoid cyst in the basal cistern. On the pathological examination, it was diagnosed as moderately differentiated SqCC and epidermoid cyst. Since primary intracranial SqCC is so rare, we performed a thorough evaluation to exclude metastatic SqCC including ear-nose-throat examination, dermatologic examination, abdominopelvic computed tomography (CT), chest CT, whole-body bone scan and positron-emission tomography (PET) scan. Because the comprehensive evaluation proved to be negative for extracranial SqCC, the patient was diagnosed as primary intracranial SqCC. There was no interval growth in the residual lesion within the right Meckel's cave at 3-months follow-up.
We presented a case of coexistence of intracranial SqCC and epidermoid cyst. The present case shows a strong enhancement of the lesion at the right CP angle in addition to the cystic lesion at the basal cistern, and the enhancing lesion increased in its size over 14 months of longitudinal follow-ups. The growing enhancing lesion later invaded the trigeminal ganglion. This case is unique in that one part, possibly the portion of SqCC, grows in size and causes discrete clinical symptoms while the rest, possibly the portion of epidermoid cyst, remains stable for years. There are very few cases in which epidermoid cyst and intracranial squamous cell carcinoma lesions coexist. Kim et al. (4) reported a case of squamous cell carcinoma in the brain stem and a cerebellopontine angle epidermoid cyst coexisting. In the case, the rapidly growth of lesion at the CPA was a clue to differential diagnosis suggesting malignancy.
Intracranial SqCC can be caused by metastases, direct extension from the cranial base and primary squamous cell carcinomas. Although rare, primary intracranial squamous cell carcinomas were classified into five categories (5): 1) Initial malignant transformation of an epidermoid cyst; 2) Malignant transformation from a remnant epidermoid cyst; 3) Malignant transformation with leptomeningeal carcinomatosis; 4) SCC carcinoma arising from other benign cysts; 5) Other malignancies arising from benign cysts. In our case, etiology of SqCC is somewhat unclear. Possibility of malignant transformation from epidermoid cyst can be raised, but coexistence of both SqCC and epidermoid cyst is more appropriate as there was solid enhancing portion from the first examination.
Previous studies showed that contrast enhancement within an epidermoid cyst on CT or MRI may indicate malignant transformation into squamous cell carcinoma (26). However, inflammation within the epidermoid cyst can also cause contrast enhancement and may be indistinguishable from tumorous condition. Also, chronic inflammatory response to repeated cyst rupture is known to attribute to malignant transformation (7). More reliable parameters would be rapid increase in size of epidermoid cyst (5) as seen in our case. The contrast enhancing lesion showed sudden dramatic growth having previously remained stable (for many years), which may be a (diagnostic) clue to differentiate from a benign epidermoid cyst.
The reports of SqCC arising from epidermoid cysts and its imaging findings have been rarely reported and DWI images were included in only three cases (489). Typically, epidermoid cysts are characterized by prolonged T1 and T2 relaxation times (low T1 signal intensity, high T2 signal intensity) as well as hyperintensity on DWI (3), because they contain both cholesterol and keratin. Compared to typical benign epidermoid cysts, malignant part of the tumor showed lower signal intensity on DWI (10). This distinguishes from other malignant lesions in the brain, where higher signal intensity on DWI (or lower value of apparent diffusion coefficient) usually indicates malignancy. But based on our case, SqCC may not exhibit diffusion restriction. Instead, the solid portion of SqCC may exhibit heterogeneity while the nonenhancing portion of epidermoid cyst exhibits homogenous diffusion restriction. But DWI heterogeneity can be nonspecific and does not necessarily indicate malignant transformation because it can also occur in the region of inflammation. This emphasizes importance of follow-up imaging in patients of epidermoid cyst with solid enhancing portion, which may become helpful than contrast enhancement or diffusion restriction on MRI.
The treatment of choice is known to be complete removal of the tumor and surveillance for recurrent malignant transformation. Nevertheless, the prognosis of primary intracranial SqCC is poor and only 1 patient survived out of 43 cases on 5-year follow-up (2). Our case did not show recurrence on short-term follow-up, but long term follow up is warranted.
In conclusion, a solid enhancing portion adjacent to a typically-looking epidermoid cyst may become a clue for coexistence of intracranial SqCC. An initial contrast enhancement and/or heterogeneous DWI may become a useful diagnostic clue, but more importantly, sudden rapid growth is important in formulating diagnosis. These findings, however, need to be further validated in a larger case series.
Figures and Tables
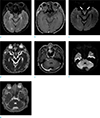 | Fig. 1Initial MRIs were taken at a local hospital, which show lobulated lesion in the basal cistern on (a) FLAIR image, and (b) contrast-enhanced T1WI shows no definite enhancing portion in the lesion. (c) DWI and (d) ADC images show diffusion restriction of the lesion (arrows). Another 1.3 cm sized enhancing lesion was noted in the right CP angle on (e) contrast-enhanced T1WI. Mild but heterogeneous diffusion restriction is shown on (f) DWI and (g) ADC (arrowheads). ADC = apparent diffusion coefficient; DWI = diffusion-weighted image; FLAIR = fluid-attenuated inversion recovery; MRI = magnetic resonance imaging; T1WI = T1-weighted image |
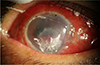 | Fig. 2Ophthalmologic examination and clinical photo show severe corneal ulceration on the right side, which was getting worse despite proper management. |
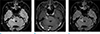 | Fig. 3Second MRIs were taken 5 months after the initial MRIs. The high signal intensity lesion on (a) FLAIR image is more prominent since the previous examination, and the enhancing lesion on (b) T1WI has increased in size as well, with minimal enhancement on (c) contrast-enhanced FLAIR image. |
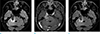 | Fig. 4Third MRIs were taken 9 months after the second MRIs or 14 months after the initial MRIs. The extent of high signal intensity lesion on (a) FLAIR image has been increased, and the size of enhancing lesion on (b) T1WI increased further, with minimal enhancement on (c) contrast-enhanced FLAIR image. |
References
1. Ahmed I, Auguste KI, Vachhrajani S, Dirks PB, Drake JM, Rutka JT. Neurosurgical management of intracranial epidermoid tumors in children. Clinical article. J Neurosurg Pediatr. 2009; 4:91–96.
2. Roh TH, Park YS, Park YG, Kim SH, Chang JH. Intracranial squamous cell carcinoma arising in a cerebellopontine angle epidermoid cyst: a case report and literature review. Medicine (Baltimore). 2017; 96:e9423.
3. Wagle WA, Jaufmann B, Mincy JE. Magnetic resonance imaging of fourth ventricular epidermoid tumors. Arch Neurol. 1991; 48:438–440.

4. Kim MS, Kim OL. Primary intracranial squamous cell carcinoma in the brain stem with a cerebellopontine angle epidermoid cyst. J Korean Neurosurg Soc. 2008; 44:401–404.

5. Hamlat A, Hua ZF, Saikali S, et al. Malignant transformation of intra-cranial epithelial cysts: systematic article review. J Neurooncol. 2005; 74:187–194.

6. Uchino A, Hasuo K, Matsumoto S, et al. Intracranial epidermoid carcinoma: CT and MRI. Neuroradiology. 1995; 37:155–158.

7. Abramson RC, Morawetz RB, Schlitt M. Multiple complications from an intracranial epidermoid cyst: case report and literature review. Neurosurgery. 1989; 24:574–578.

8. Nakao Y, Nonaka S, Yamamoto T, et al. Malignant transformation 20 years after partial removal of intracranial epidermoid cyst--case report. Neurol Med Chir (Tokyo). 2010; 50:236–239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download