Abstract
Purpose
This study examined the effects of beraprost sodium on digital infrared thermal images in patients with peripheral arterial disease caused by type 2 diabetes mellitus.
Materials and Methods
Twenty-five diabetic patients with peripheral arterial disease were treated with beraprost sodium in a prospective, multicenter, cohort study from February 2013 to December 2014. Beraprost sodium (40 µg) was administered orally 3 times daily (120 µg/day) for 6 months. The visual analogue scale (VAS) and digital infrared thermal imaging (DITI) were performed to compare the blood flow improvement between before and after dosing.
Results
Among the 25 patients included in the evaluation, 22 patients completed the study. A significant increase in body temperature was observed in the front and left side, particularly in the plantar side in DITI compared to that before and after administration. An increase in body temperature was observed at the frontal part from 28.1°C±2.3°C to 29.1°C±2.1°C (p=0.021), at the left side from 27.8°C±2.4°C to 28.6°C±1.9°C (p=0.028), at the plantar part at 24.0°C±1.5°C, and at the plantar part at 27.1°C±2.4°C (p<0.01). The VAS decreased significantly from 5.4±1.3 to 2.7±2.0 after 6 months of treatment (p<0.01).
Go to : 
References
1. Diabetes fact sheet in Korea 2016 [Internet]. Seoul: Korean Diabetes Association;[cited 2018 Jun 2]. Available from:. http://www.diabetes.or.kr/pro/news/.
2. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005; 293:217–28.

3. Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care. 2011; 34:1695–700.

4. Yoon HS, Choi WJ, Sung IH, Lee HS, Chung HJ, Lee JW. Effects of beraprost sodium on subjective symptoms in diabetic patients with peripheral arterial disease. Clin Orthop Surg. 2013; 5:145–51.

5. Demolis JL, Robert A, Mouren M, Funck-Brentano C, Jaillon P. Pharmacokinetics and platelet antiaggregating effects of beraprost, an oral stable prostacyclin analogue, in healthy volunteers. J Cardiovasc Pharmacol. 1993; 22:711–6.

6. Lièvre M, Morand S, Besse B, Fiessinger JN, Boissel JP. Oral beraprost sodium, a prostaglandin I(2) analogue, for intermittent claudication: a double-blind, randomized, multicenter controlled trial. Beraprost et Claudication Intermettente (BERCI) research group. Circulation. 2000; 102:426–31.
7. Nony P, Ffrench P, Girard P, Delair S, Azoulay S, Girre JP, et al. Platelet-aggregation inhibition and hemodynamic effects of beraprost sodium, a new oral prostacyclin derivative: a study in healthy male subjects. Can J Physiol Pharmacol. 1996; 74:887–93.

8. Uchikawa T, Murakami T, Furukawa H. Effects of the antiplatelet agent cilostazol on peripheral vascular disease in patients with diabetes mellitus. Arzneimittelforschung. 1992; 42:322–4.
9. Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE Jr, Bortey EB, et al. A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial. Arch Intern Med. 1999; 159:2041–50.
10. Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000; 109:523–30.

11. Dawson DL, Cutler BS, Meissner MH, Strandness DE Jr. Cilostazol has beneficial effects in treatment of intermittent claudication: results from a multicenter, randomized, prospective, double-blind trial. Circulation. 1998; 98:678–86.

12. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006; 47:921–9.

13. Belch JJ, Bell PR, Creissen D, Dormandy JA, Kester RC, McCollum RD, et al. Randomized, double-blind, placebocontrolled study evaluating the efficacy and safety of AS-013, a prostaglandin E1 prodrug, in patients with intermittent claudication. Circulation. 1997; 95:2298–302.
14. Müller-Bühl U, Diehm C, Krais T, Zimmermann R, Mörl H, Eckstein HH. Clinical effects of intravenous iloprost in patients with intermittent claudication. Eur J Clin Pharmocol. 1987; 33:127–31.
15. Friedman R, Mears JG, Barst RJ. Continuous infusion of prostacyclin normalizes plasma markers of endothelial cell injury and platelet aggregation in primary pulmonary hypertension. Circulation. 1997; 96:2782–4.

17. Goya K, Otsuki M, Xu X, Kasayama S. Effects of the prostaglandin I2 analogue, beraprost sodium, on vascular cell adhesion molecule-1 expression in human vascular endothelial cells and circulating vascular cell adhesion molecule-1 level in patients with type 2 diabetes mellitus. Metabolism. 2003; 52:192–8.

18. Galiè N, Manes A, Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Med. 2003; 2:123–37.

19. Ueno Y, Koike H, Nakamura Y, Ochi Y, Annoh S, Nishio S. Effects of beraprost sodium, a prostacyclin analogue, on diabetic neuropathy in streptozotocininduced diabetic rats. Jpn J Pharmacol. 1996; 70:177–82.

20. Aso Y, Tayama K, Takanashi K, Inukai T, Takemura Y. Changes in skin blood flow in type 2 diabetes induced by prostacyclin: association with ankle brachial index and plasma throbomodu-lin levels. Metabolism. 2001; 50:568–72.
Go to : 
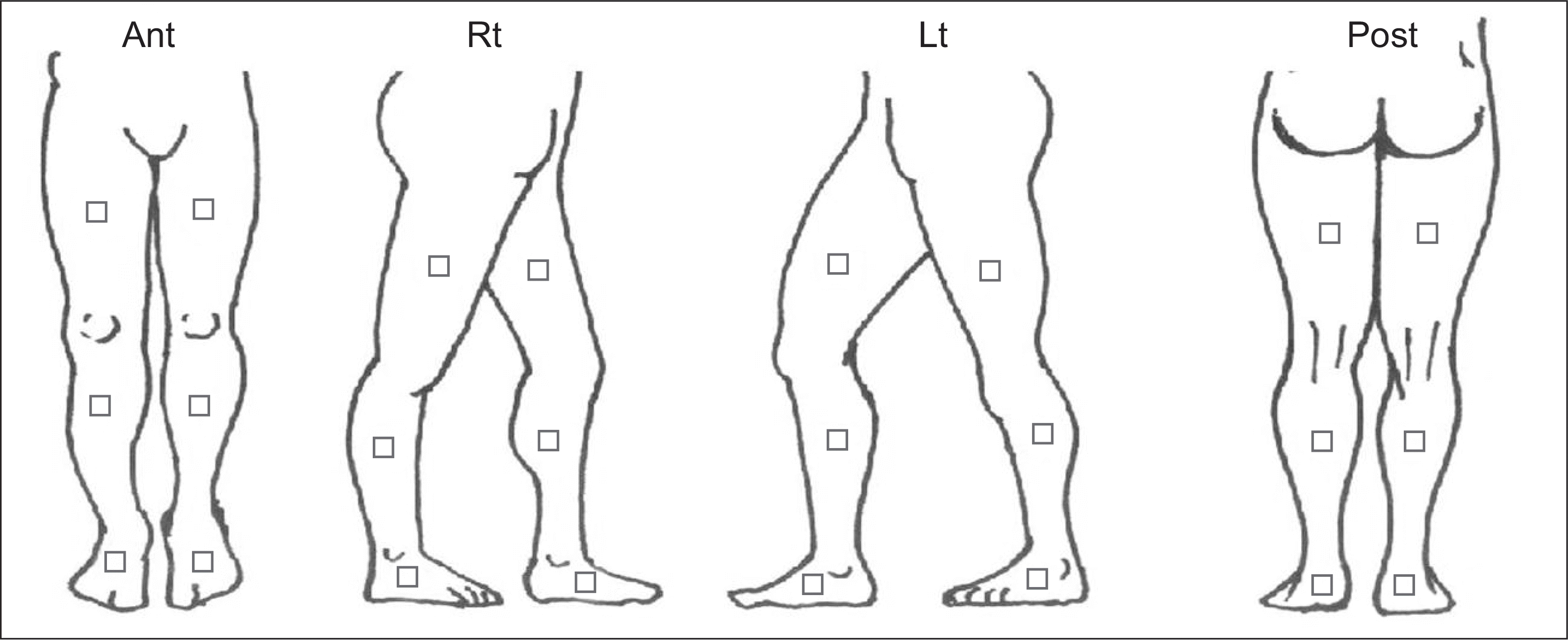 | Figure 1.A schematic diagram of digital infrared thermal imaging (DITI). DITI was performed on each of four photographs of the anterior (Ant), posterior (Post), left (Lt), and right (Rt) sides of the lower limbs. |
 | Figure 2.A 63-year-old male digital infrared thermal imaging (DITI) before medication. (A) Anterior part (Ant), posterior part (Post), right side (Rt), and left side (Lt). (B) Plantar side. |
 | Figure 3.A 63-year-old male digital infrared thermal imaging (DITI) after 6 month taking medicine. Compared to Fig. 2, there is an increase in body temperature. (A) Anterior part (Ant), posterior part (Post), right side (Rt), and left side (Lt). (B) Plantar side. |
Table 1.
Patients Characteristics
| Characteristic | Value |
|---|---|
| No. of patients | 22 |
| Age (yr) | 59.3±6.3 |
| Male:female | 13:9 |
| Height (cm) | 164.4±8.1 |
| Weight (kg) | 65.2±10.1 |
| Body mass index (kg/m2) | 23.5±3.2 |
| Diabetes duration (yr) | 13.3±8.0 |
Table 2.
Difference in Laboratory Findings after Beroprost Sodium Administration
| Laboratory variable | Before | After | p-value |
|---|---|---|---|
| AST/ALT (IU/L) | 24/32 | 26/28 | 0.915 |
| BUN/Cr (mg/dL) | 24/1.1 | 25/1.2 | 0.780 |
| Glucose (mg/dL) | 277.7±24.4 | 283.3±18.8 | 0.321 |
| HbA1c (%) | 7.8±1.6 | 7.7±1.4 | 0.930 |
Table 3.
Difference in Thermography and Visual Analogue Scale after Beroprost Sodium Administration




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download