Abstract
Induction therapy with basiliximab is widely administered after kidney transplantation to prevent acute rejection. Herein, we report a case of non-cardiogenic pulmonary edema induced by basiliximab. To the best of our knowledge, such case has not been reported to date in Korea. A 54-year-old man with polycystic kidney disease received kidney transplantation. As induction therapy, he was prescribed basiliximab. On day 4, the second dose of basiliximab was administered. The patient complained of acute hypoxia 23 hours later, which led to circulatory collapse. He was discharged 3 weeks later with stable renal function. Pulmonary edema was presumed to have been caused by increased pulmonary capillary permeability. A possible hypothesis for this event occurring after the second basiliximab injection is steroid-related effects. Non-cardiogenic pulmonary edema is a complication that might occur after basiliximab induction therapy. Physicians should be aware of this potentially life-threatening complication.
Acute rejection is one of the most important predictors of long-term prognosis after renal transplantation(1). Induction therapy, defined simply as the short-term use of an immunosuppressive agent, most commonly a T-lymphocyte-depleting rabbit-derived anti-thymocyte globulin or an interleukin-2 receptor antagonist (IL2RA), is administered to reduce acute rejection and improve long-term graft survival. The use of selective induction agents, such as IL2RA, basiliximab, and daclizumab, has reduced the frequency of acute rejection(2). Because of the relatively fewer adverse effects and higher convenience than those of other induction therapy agents, basiliximab is widely used in immunologically low-risk recipients(3). Pulmonary edema as an adverse effect of basiliximab has not been reported in Korea. Herein, we report a case of non-cardiogenic pulmonary edema induced by basiliximab during the induction
therapy.
A 54-year-old male patient with autosomal dominant polycystic kidney disease and hypertension was advised a preemptive kidney transplantation. The donor was his wife, a 54-year-old woman, with human leukocyte antigen mismatch 4/6, ABO compatibility, complement-dependent cytotoxicity cross-match negativity, and 0% panel reactive antibodies. Pre-operative evaluation of the patient was unremarkable, except for his kidney enlargement (Fig. 1). Pre-operative laboratory findings were as follows; hemoglobin 8.6 g/dL, platelets 269×109/L, protein 6.9 g/dL, white blood cells 6.30×109/L, albumin 3.7 g/dL, aspartate transaminase 15 U/L, alanine transferase 15 U/L, blood urea nitrogen 61.5 mg/dL, creatinine 5.02 mg/dL, sodium 143 mmol/L, potassium 4.9 mmol/L, and chloride 111 mmol/L. There was no active lung lesion on pre-operative plain chest radiograph (Fig. 2). Preoperative echocardiography results were as follows: ejection fraction 66%, no regional wall motion abnormality with normal global left ventricular (LV) systolic function. He received 20 mg of basiliximab as induction therapy and prednisone, tacrolimus, and mycophenolate mofetil as maintenance therapy. A 1,000 mg of methylprednisolone was to be administered on the day of surgery, and the dose was then to be reduced by half each day thereafter, until a maintenance dose of 1 mg/kg was reached. Due to limited abdominal space, his enlarged kidneys were removed by laparoscopic simple nephrectomy on the left side and hand-assisted laparoscopic nephrectomy on the right side before the kidney transplantation. The surgery lasted 7 hours and 30 minutes. The transplanted kidney had 104 minutes of cold ischemic time and 33 minutes of warm ischemic time. On the first post-operative day, his vital signs were stable and the urine output was preserved at >10.1 mL/kg/hour. Post-operative 24-hour creatinine was 2.43 mg/dL. On the 4th post-operative day, the second dose of basiliximab was administered as per the treatment schedule. The patient was administered 1 g of methylprednisolone during the first dose of basiliximab and 60 mg at the second administration. Twenty-three hours later, he suddenly complained of shortness of breath and required more than 15 L/min of oxygen via a reservoir mask. Additionally, circulatory collapse was noticed with a systolic pressure of 50 mmHg. Arterial blood analysis results were as follows: pH 7.08, PaCO2 45 mmHg, PaO2 58 mmHg, O2 saturation 76%, and bicarbonate 13.3 mmol/L; and his laboratory findings were as follows: white blood cells 8.90×109/L, eosinophil 1.7%, and lactate dehydrogenase 155 IU/L. The chest radiograph showed acute pulmonary infiltration in both lung fields (Fig. 2). The central venous pressure was 8 cmH2O. Results from electrocardiography were within normal limits (Fig. 3). Chest computed tomography showed increased interstitial infiltrates in the dependent portion of both lower lung fields (Fig. 4). As he failed to respond to usual therapies, including high doses of inotropics and vasopressors with mechanical ventilation, he was put on extracorporeal membrane oxygenation (ECMO, V-A mode) to maintain his oxygen saturation and blood pressure. There was no evidence of infection (C-reactive protein 0.98 mg/dL, procalcitonin 0.5 ng/mL, blood and urine culture were negative). However, except for methylprednisolone, tacrolimus and mycophenolate mofetil were temporarily stopped and empirical antibiotics were administrated as septic shock. With an ejection fraction of 50%, global hypokinesia on echocardiography, no definite findings on electrocardiography, and normal cardiac enzyme levels (creatine kinase-MB [CKMB] 2.8 ng/mL, troponin I <0.0041 ng/mL), cardiogenic shock was not suspected. Two days later, his blood pressure returned to normal and he was weaned off the ECMO. Tacrolimus and mycophenolate mofetil were administered again. After 4 days of supportive care, he recovered from the acute pulmonary event and was weaned off the ventilator. The follow-up echocardiography showed an ejection fraction of 60% and no regional wall motion abnormality with normal LV systolic function and the follow-up cardiac enzyme levels (CKMB 4.1 ng/mL, troponin I <0.0039 ng/mL) were normal. Three weeks later, he returned home safely. His last serum creatinine was 1.59 mg/dL and spot urine protein/creatinine ratio was 114.9 mg/gCr. Two months later, his serum creatinine 1.76 and spot urine protein/creatinine ratio was 68.4 mg/gCr (Fig. 5).
Acute rejection plays an important role in determining the outcome of kidney transplants(1). Induction therapy is administered to prevent acute rejection and improve long-term graft survival. The choice of induction therapy depends on the immunological risk of the recipient, the underlying disease, or the choice of maintenance immunosuppression therapy. Basiliximab and chimeric monoclonal antibodies are used in combination with other immunosuppressants to prevent acute rejection reactions in adult and pediatric kidney transplant patients. It blocks the binding of serum interleukin-2 (IL-2) to CD25, inhibiting the proliferation of activated T-cells and subsequently releasing cytokines. Basiliximab (20 mL) is administered twice, on day 1 after transplantation and on day 4 after transplantation(4). It reduces the frequency of acute rejection and has been proven effective in reducing the severity of acute rejections in kidney transplant patients(2).
Because of the relatively fewer side effects and its ease of use compared with other induction therapy agents, basiliximab is widely used in immunologically low-risk recipients(3). Unlike OKT-3, basiliximab was initially thought to be free from immune-mediated side effects(456). However, basiliximab-related hypersensitivity was recently reported. Type 1 anaphylactic reactions to basiliximab have been described in young women who successfully received humanized monoclonal antibodies as induction agents for renal transplantation(7). In a similar report by the Mayo Clinic, infliximab, a chimeric human/murine monoclonal antibody (mAb) against tumor necrosis factor α (TNF-α), was found to be involved in acute respiratory distress syndrome triggered by type IV anaphylaxis(8). Patients suffering an anaphylactic reaction usually develop hypotension and allergic signs such as pruritic rash, swelling of the tongue, and flushing of the skin within minutes or hours following basiliximab injection(910). However, our patient had no allergic signs and symptoms. Moreover, there was a delay of several hours between basiliximab injection and the onset of symptoms. The mechanism of pulmonary edema in our patient is presumed to be an increase in pulmonary capillary permeability resulting from cytokine release. Unlike that of type 1 hypersensitivity reactions, the expression of respiratory symptoms is rather insidious, developing within 48 hours. The pathogenesis of acute lung injury in transplant recipients administered monoclonal antibodies might include cytokine release from the activated T-cells and sequestration of neutrophils within the pulmonary capillary beds. The smaller diameter of the pulmonary capillaries might increase the possibility of neutrophil deformation and increase the regulation of adherent capillaries to neutrophils and vascular endothelium, leading to leakage of neutrophils into the alveolar tissue(1011). The activation of neutrophils by an inflammatory mediator results in the release of L-selectin from their surface and in the upward regulation of B2 integrins, which interact with endothelial intercellular adhesion molecule-1 to cause translocation of neutrophils into the alveoli(12).
A possible hypothesis for the occurrence of this event after the second basiliximab injection is the use of steroids. Glucocorticoids inhibit the expression and action of most cytokines(13). Mitsuta et al.(14) have demonstrated that pre-operative corticosteroid therapy significantly suppressed both IL-5 and TNF-α production. The first basiliximab injection would have been accompanied by high concentrations of methylprednisolone, which would have led to suppression of cytokine production. With the relatively small doses of methylprednisolone being administered during the second basiliximab injection, cytokine production would have not been adequately suppressed, which could have led to pulmonary edema.
We reported a life-threatening episode of non-cardiogenic pulmonary edema and shock in a 54-year-old patient following administration of the second dose of basiliximab. To our knowledge, non-cardiogenic pulmonary edema as an adverse effect of basiliximab has not been reported in Korea. This report does not show a clear causal relationship between basiliximab and pulmonary edema. However, we were able to exclude other causes of pulmonary edema or infiltration, such as cardiogenic and infectious causes. Non-cardiogenic pulmonary edema, although rare, might occur as a complication after basiliximab induction therapy. Physicians need to be aware of this potentially life-threatening complication. This might improve graft survival in kidney transplantation.
Figures and Tables
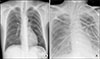
Fig. 2
The chest radiograph showed no active lung lesion (A), and acute pulmonary infiltration in both lung fields (B).
References
1. Pirsch JD, Ploeg RJ, Gange S, D'Alessandro AM, Knechtle SJ, Sollinger HW, et al. Determinants of graft survival after renal transplantation. Transplantation. 1996; 61:1581–1586.

2. Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004; 4:378–383.

3. Al Najjar A, Etienne I, Le Pogamp P, Bridoux F, Le Meur Y, Toupance O, et al. Long-term results of monoclonal anti-Il2-receptor antibody versus polyclonal antilymphocyte antibodies as induction therapy in renal transplantation. Transplant Proc. 2006; 38:2298–2299.

4. Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group. Lancet. 1997; 350:1193–1198.

5. Goldman M, Abramowicz D, De Pauw L, Alegre ML, Widera I, Vereerstraeten P, et al. OKT3-induced cytokine release attenuation by high-dose methylprednisolone. Lancet. 1989; 2:802–803.

6. Gaston RS, Deierhoi MH, Patterson T, Prasthofer E, Julian BA, Barber WH, et al. OKT3 first-dose reaction: association with T cell subsets and cytokine release. Kidney Int. 1991; 39:141–148.

7. Leonard PA, Woodside KJ, Gugliuzza KK, Sur S, Daller JA. Safe administration of a humanized murine antibody after anaphylaxis to a chimeric murine antibody. Transplantation. 2002; 74:1697–1700.

8. Riegert-Johnson DL, Godfrey JA, Myers JL, Hubmayr RD, Sandborn WJ, Loftus EV Jr. Delayed hypersensitivity reaction and acute respiratory distress syndrome following infliximab infusion. Inflamm Bowel Dis. 2002; 8:186–191.

9. Barros VR, Rocha V, Garcia VD, Garcia CD. Anaphylactic shock after retreatment with basiliximab. Transplant Proc. 2003; 35:579.

10. Wouters KM, Lane MH, Walker I. Acute hypersensitivity reaction on re-exposure to basiliximab in an infant undergoing heart transplantation. Paediatr Anaesth. 2008; 18:806–807.

11. Steinberg KP, Hudson LD. Acute lung injury and acute respiratory distress syndrome. The clinical syndrome. Clin Chest Med. 2000; 21:401–417.
12. Downey GP, Dong Q, Kruger J, Dedhar S, Cherapanov V. Regulation of neutrophil activation in acute lung injury. Chest. 1999; 116:1 Suppl. 46S–54S.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download