Dear Editor,
Levetiracetam (LEV) is an antiepileptic drug with a low toxicity in epilepsy treatment.1 There have been recent reports of adults exhibiting LEV-associated increases in alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (γ-GT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and creatine kinase (CK).234 We prospectively investigated changes in liver enzymes and CK in children with epilepsy receiving LEV monotherapy.
The study population consisted of 26 ambulatory children [17 females; age 6.3±4.1 years (mean±SD), age range 1–15 years] who had received LEV monotherapy for new-onset epilepsy. Twenty-one children suffered from focal epilepsy and five from generalized epilepsy. All of the children performed their normal daily activities and did not take any other medication known to interfere with liver function. Informed parental consents were obtained, and the study was approved by the local institutional review board. Serum ALT, AST, LDH, γ-GT, ALP, and CK levels were measured as parameters before and after 2 and 6 months of LEV treatment.
Data were analyzed using the Statistical Package for Social Sciences (SPSS version 23.0, IBM Corp., Armonk, NY, USA). The Wilcoxon signed-rank test was used to assess the statistical significance of changes in the parameters between baseline and after treatment with LEV. The correlations between the parameters and the LEV dose were assessed using Spearman's correlation coefficient.
The pubertal stage did not change in any of the children during the 6-month follow-up. The ALP concentration was significantly increased after 2 and 6 months of treatment, and the γ-GT concentration was significantly increased after 2 months of treatment (Table 1). There were no significant alterations in the other parameters evaluated. All measured γ-GT levels were within normal limits before and after the initiation of LEV therapy. ALP concentrations were within normal limits before treatment, while two children at 2 months and three children at 6 months had values above the age-specific upper reference limit. The drug dose was 16.5±2.9 mg/kg after 2 months of treatment and 21.2±9.9 mg/kg after 6 months of treatment. No association was found between γ-GT or ALP and the LEV dose after 2 and 6 months of treatment.
LEV does not interact with the cytochrome P450 system and is the drug of first choice for patients with liver disease.1 In the present study, the serum γ-GT level in 26 children had increased significantly after 2 months of LEV monotherapy. An unexpected significant increase in γ-GT was reported in a 58-year-old woman with epilepsy after 4–12 months of LEV monotherapy.4 However, the γ-GT levels remained within normal limits throughout the present study. Whether the above finding indicates the early (but not persistent) induction of a hepatic response by LEV exposure needs further investigation.
The mechanism underlying the significant increase in serum ALP after 2 and 6 months of LEV treatment is unknown. Since LEV has been implicated in both liver and bone disease,5 further prospective studies evaluating ALP isoenzymes and the vitamin D status are needed to clarify the origin of this persistent alteration in ALP levels. There is a particularly interesting report of probable LEV-related serum ALP elevation in a 10-month-old girl, which decreased to normal after LEV discontinuation.6 However, the absence of an association between ALP and γ-GT changes after LEV treatment in our study implies that the significant increase in ALP levels after 2 and 6 months of treatment was probably not attributable to liver disease.
In conclusion, LEV monotherapy may affect the liver enzyme level in children with epilepsy. Further prospective studies are needed to investigate the origin and clinical significance of the changes reported here, and whether these parameters should be monitored in children taking LEV.
References
1. Lyseng-Williamson KA. Levetiracetam: a review of its use in epilepsy. Drugs. 2011; 71:489–514. PMID: 21395360.
2. Sethi NK, Sethi PK, Torgovnick J, Arsura E, Cukierwar F. Asymptomatic elevation of liver enzymes due to levetiracetam: a case report. Drug Metabol Drug Interact. 2013; 28:123–124. PMID: 23420283.

3. Shahbaz N, Younus SM, Khan SA, Ain Q, Khan MA, Memon MH. Levetiracetam induced increase in creatine phosphokinase levels. J Coll Physicians Surg Pak. 2017; 27:S63–S64. PMID: 28302251.
4. Broli M, Provini F, Naldi I, Bisulli F, Sama C, Baruzzi A, et al. Unexpected gamma glutamyltransferase rise increase during levetiracetam monotherapy. Epileptic Disord. 2010; 12:81–82. PMID: 20159672.

5. Hakami T, O'Brien TJ, Petty SJ, Sakellarides M, Christie J, Kantor S, et al. Monotherapy with levetiracetam versus older AEDs: a randomized comparative trial of effects on bone health. Calcif Tissue Int. 2016; 98:556–565. PMID: 26842957.

6. Xiong N, Hou L, Lu N, Mohamed AA, Wang T, Huang Y. Probable levetiracetam-related serum alkaline phosphatase elevation. BMC Neurol. 2012; 12:97. PMID: 22994584.

Table 1
Serum concentrations of ALT, AST, LDH, γ-GT, ALP, and CK in 26 epileptic children before and after 2 and 6 months of levetiracetam monotherapy
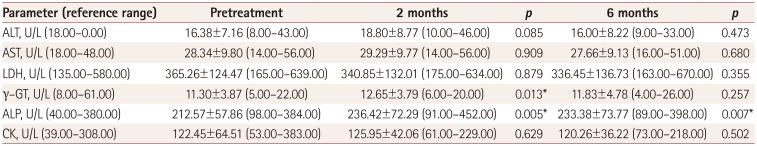
Data are mean±SD (range) values. p values are for comparisons with the pretreatment levels.
*Statistically significant differences (p<0.05).
ALP: alkaline phosphatase, ALT: alanine aminotransferase, AST: aspartate aminotransferase, CK: creatine kinase, γ-GT: gamma-glutamyltransferase, LDH: lactate dehydrogenase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download