This article has been
cited by other articles in ScienceCentral.
Abstract
Background and Purpose
Cheyne-Stokes respiration (CSR) is frequently observed in patients with acute stroke. There have been conflicting opinions about the associations of CSR with the location and size of the lesion. We aimed to better define the clinical relevance and pathogenesis of CSR in acute stroke.
Methods
We investigated patients who had been admitted with acute ischemic stroke and received an overnight sleep apnea test. We collected data on demographics, risk factors, etiologic subtypes, initial vital signs, clinical course of the stroke, and parameters associated with respiratory events during the sleep apnea test. We performed a multivariate logistic regression analysis to determine the factors associated with CSR.
Results
Among 182 patients, 35 patients showed CSR in sleep apnea testing. Large-artery atherosclerosis or cardioembolism, bilateral hemispheric involvement, atrial fibrillation, low left-ventricle ejection fraction (LVEF), and left atrium (LA) enlargement were all associated with the presence of CSR. Multivariate analysis revealed that the previous modified Rankin Scale (mRS) score, bilateral hemispheric involvement, low LVEF, and LA enlargement were significantly associated with CSR. Subgroup analysis with large-artery atherosclerosis without cardiac disease revealed that the previous mRS score is the only independent factor associated with CSR.
Conclusions
CSR frequently occurs in strokes involving large arteries or due to cardioembolism, regardless of the location and severity of the stroke. Predisposing conditions such as preexisting neurologic disability, low LVEF, and LA enlargement are associated with CSR in acute stroke.
Keywords: Cheyne-Stokes respiration, stroke, left-atrium enlargement
INTRODUCTION
Both obstructive sleep apnea (OSA) and central sleep apnea (CSA) occur frequently in patients with acute ischemic stroke. Cheyne-Stokes respiration (CSR), which is a form of periodic CSA, is observed in 6–72% patients with acute stroke.
12345678 In general, CSR appears immediately after the onset of the acute stroke, and gradually improves with time.
259
There has been a fair amount of research on stroke and OSA, but few studies have investigated the CSA that is seen in patients with stroke. CSR was first described by Cheyne
10 in 1,818 in a patient who presented with both a stroke and heart failure. An association with the medulla—which acts as the respiratory center—was suspected previously, but it was noted that medullary lesions would manifest as ataxic breathing rather than periodic breathing, and it was subsequently reported that this appeared more commonly in bilateral supramedullary lesions or large or extensive strokes.
11112 However, the results obtained in studies of the location prone to the development of CSA and of the size and type of stroke were inconsistent. There have also been differences in opinion on how CSA affects the outcome of strokes.
1314 Following Stokes suggesting the association between CSR and cardiac disease in 1854,
15 it is now well known that CSR frequently occurs in patients with congestive heart failure, and CSR has been reported to be associated with higher mortality in heart failure.
16 It was recently shown that CSR is associated with a low left-ventricle ejection fraction (LVEF) even in patients with stroke who do not have overt symptoms of heart failure.
17
CSR characteristically alternates between hypoventilation and hyperventilation in a crescendo-decrescendo fashion, and this breathing pattern reflects the instability of the ventilatory control system.
18 Although Cheyne and Stokes had mainly observed CSR in imminent death, it is not uncommon for CSR to also be detected on a respirogram in patients who are not critically ill. The mechanism underlying CSR in heart failure has been explained as a delay in the recognition and correction of hypo- and hyperventilation as a result of delayed circulation, leading to larger changes in blood gas and respiratory overcompensation. However, other factors besides circulation delay may be involved, and the mechanism underlying CSR in stroke in the absence of heart failure remains unclear.
18
The objective of this study was to identify factors associated with CSR in a large cohort of patients with acute ischemic stroke and to deduce the mechanism underlying CSR.
METHODS
This study involved patients who had been admitted to the Department of Neurology at Kangwon National University Hospital over a 22-month period (from May 2014 to February 2016) under a diagnosis of acute ischemic stroke that had occurred within 1 week of the date of admission. The Gangwon Comprehensive Stroke Center (CSC) has been operating under the Ministry of Health and Welfare of Korea as part of the Kangwon National University Hospital since 2009, and has been prospectively collecting and registering all patient data. We utilized the stroke database of Gangwon CSC for selecting our study subjects and collecting clinical information.
We collected data on variables such as the patient's sex and age, as well as risk factors for stroke such as a previous history of stroke, hypertension, diabetes mellitus, hyperlipidemia, body mass index, atrial fibrillation, and heart failure. The severity of the neurologic deficits at the time of the visit was assessed using the National Institutes of Health Stroke Scale (NIHSS). The degree of disability was evaluated using the modified Rankin Scale (mRS).
We classified the etiologic subtypes of ischemic stroke into large-artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardioembolism (CE), stroke of other determined etiology, and stroke of undetermined etiology,
19 according to the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST).
Based on the anatomical location of the lesion, we also classified the strokes based on their location into the left hemisphere, right hemisphere, or both, and supratentorial, infratentorial, or both, and into 10 groups according to the symptomatic vascular territories, with cases of lesions involving 2 or more territories classed as multiple lesions.
We collected data on blood pressure at the time of admission as a cardiovascular indicator, as well as the left atrium (LA) size (mm), left-atrium volume index (LAVI) (mL/m2), LVEF (%), and regional wall-motion abnormality from transthoracic echocardiography performed during admission. LAVI was calculated as dividing the maximum LA volume by the body surface area. Cases with moderate-to-severe valvular dysfunction in transthoracic echocardiography were classified as significant valvular disease.
Sleep apnea test was performed during the admission using a portable machine (Embletta ×10; Embla-Medcare, Reykjavik, Iceland). This included the use of transnasal airflow sensors, chest and abdominal belts to detect respiratory motion, posture sensors, and oximeters. We collected data on the presence of CSR, respiratory parameters, and the time from the stroke onset until performing the sleep apnea test.
CSR was defined in accordance with version 2.3 of the 2016 American Academy of Sleep Medicine criteria,
20 if both of the following were met:
a) Three or more episodes of consecutive central apneas and/or central hypopneas, separated by a crescendo-decrescendo change in breathing amplitude with a cycle length of at least 40 seconds.
b) Five or more central apneas and/or central hypopneas per hour of sleep associated with a crescendo-decrescendo breathing pattern recorded over at least 2 hours of monitoring.
A total of 529 patients with acute ischemic stroke had been admitted to the Gangwon CSC during the study period, among of which sleep apnea tests were applied to 185 patients. Sleep apnea test was routinely recommended to all patients with acute ischemic stroke, but this could not be applied to all of the patients due to cost and some cases of poor patient condition. The sleep study could not be performed in patients with a nasogastric or endotracheal tube. Two patients could not complete the sleep apnea test, resulting in a loss of data, hence those two were excluded from the study. One patient presented central periodic apnea, but with snoring and without the typical crescendo-decrescendo respiratory pattern, and therefore could not be classified depending on CSR. In summary, a study analysis was performed from the data obtained from a total of 182 patients.
We compared clinical indicators between the CSR and non-CSR groups. Continuous variables were analyzed as mean±standard-deviation values, whereas categorical variables were analyzed as number and percentage values. The continuous variables were analyzed statistically using the independent t-test, and categorical variables were analyzed using Pearson's chi-square test, and occasionally Fisher's exact test. Multiple regression analysis was used to identify independent clinical factors associated with CSR. A subgroup analysis was performed in the same manner to analyze the direct effects of the stroke itself, excluding cardiogenic causes, which are known to cause CSR. This subgroup consisted of patients with the stroke etiology of LAA, no atrial fibrillation or any significant valvular disease, and an LVEF of 50% or higher.
Variables with a p value of less than 0.1 in the univariate analysis were included in the multivariable analysis. Since the cases of stroke due to either atrial fibrillation or CE overlapped, only CE was included in the multivariate analysis. Since all of the patients with heart failure showed a decreased LVEF, only LVEF was included in the multivariate analysis. Regional wall-motion abnormality was also significantly associated with a decreased LVEF (p<0.001), and hence was not included in the multivariate analysis. Since both LA size and LAVI are indicators of LA enlargement, only LAVI was included in the multivariate analysis. A previous mRS score and a previous history of stroke were also strongly associated with each other, hence only one of these variables was included in the multivariate analysis. A p value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS software (version 23.0, IBM Corp., Armonk, NY, USA).
The study was approved by the Institutional Review Board of Kangwon National University Hospital (IRB No. KNUH-2016-03-007-001).
RESULTS
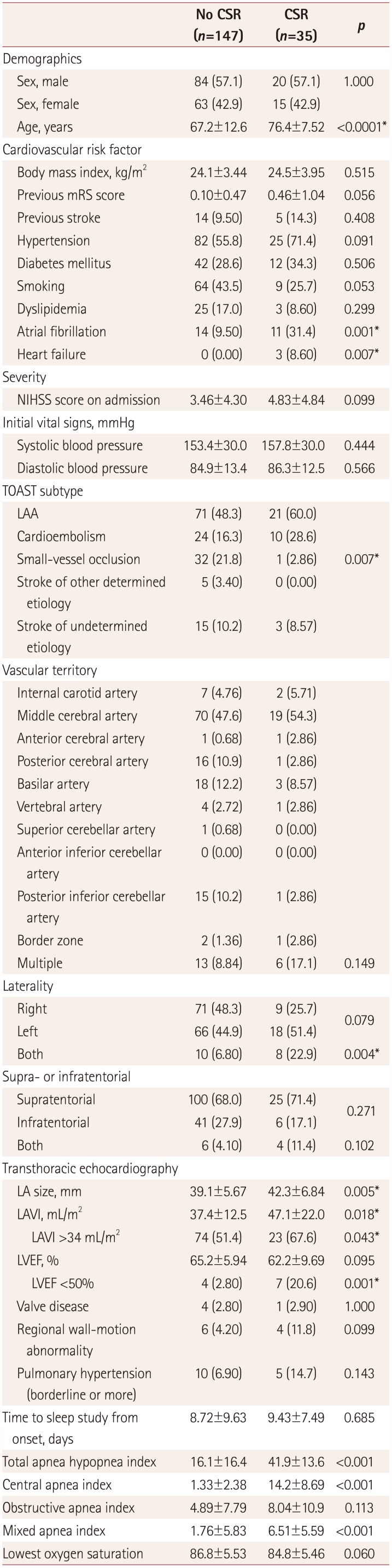
CSR was present in 35 (19.1%) of the 182 included patients. The mean time from the onset of the acute stroke until the day of the sleep apnea test was 9.4 days in the CSR group and 8.7 days in the non-CSR group, which was not significantly different.
Univariate analysis
The patients were about 9 years older in the CSR group (76.4±7.52 years) than in the non-CSR group (67.2±12.6 years, p<0.0001). In addition, the frequencies of atrial fibrillation (31.4% vs. 9.50%, p=0.001) and heart failure (8.60% vs. 0.00%, p=0.007) were higher in the CSR group.
When classified according to the TOAST classification, there was a significant difference based on the presence of CSR. Only one patient in the SVO group had CSR (2.86%), which was significantly less frequent than in the other groups (p=0.007). This patient had complained of dyspnea upon exertion of New York Heart Association class II, and was observed to have pitting edema at 1 month after the onset of stroke. There were no significant coronary lesions and the LVEF was normal, but an echocardiogram showed high ratio of the early transmitral filling velocity to the early diastolic tissue velocity at the septal mitral annulus (E/e′) (19.02), which improved after administering diuretics. Hence the patient was diagnosed as heart failure with preserved LVEF.
There was no significant association between the presence of CSR and the location of the lesion in terms of vascular territory, supra- or infratentorial, or laterality. Having bilateral hemispheric lesions was significantly associated with CSR (p=0.004).
Echocardiography was performed in 179 patients (98%). Among the echocardiographic indicators, the LA size (42.3±6.84 mm vs. 39.1±5.67 mm,
p=0.005) and LAVI (47.1±22.0 mL/m
2 vs. 37.4±12.5 mL/m
2,
p=0.018) were larger in the CSR group. Low LVEF (<50%) was more common in the CSR group (
p=0.001) (
Table 1).
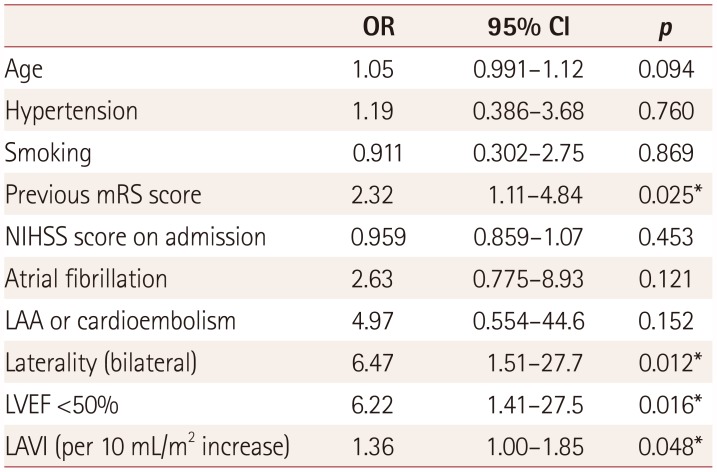
Multivariate analysis
A multivariate analysis was performed on the following factors that differed between the two groups with a
p value of less than 0.1 in the univariate analysis based on the presence of CSR: age, hypertension, smoking status, previous mRS score, NIHSS score at the time of admission, bilateral lesions, LAVI, and an LVEF of less than 50%. The previous mRS score [odds ratio (OR)=2.32,
p=0.025], bilateral lesions (OR=6.47,
p=0.012), increased LAVI (OR=1.36 for every 10 mL/m
2 increase,
p=0.048), and an LVEF of less than 50% (OR=6.22,
p=0.016) were significantly associated with CSR (
Table 2).
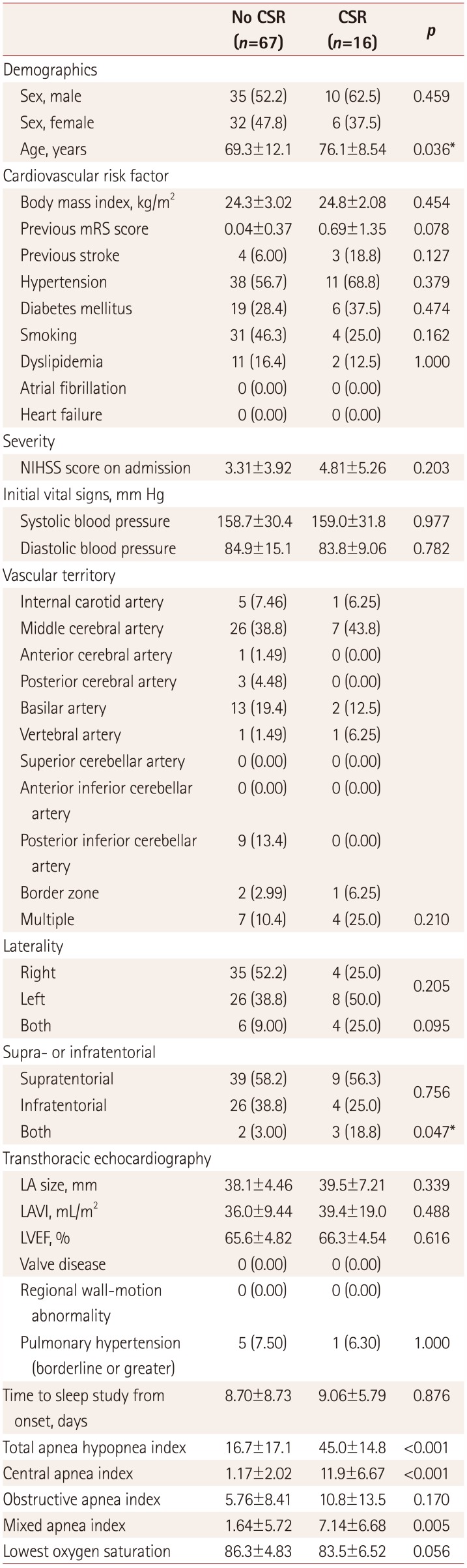
Subgroup analysis
A subgroup analysis of the 83 patients in the LAA group who did not have atrial fibrillation or any significant valvular disease with a normal LVEF revealed CSR in 16 patients (19.3%). An analysis of the factors associated with CSR showed that the patients in the CSR group were older (76.1±8.54 vs. 69.3±12.1,
p=0.036) and had both supra- and infratentorial lesions more frequently (18.8% vs. 3.00%,
p=0.047) (
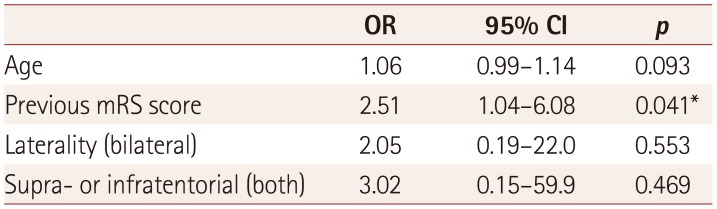
Table 3). A multivariate analysis of the factors that differed significantly with a
p value of less than 0.1 in the univariate analysis between the two groups—age, previous mRS score, both supra- and infratentorial lesions, and bilateral lesions—found that only the previous mRS score was a significant factor (OR=2.51,
p=0.041) (
Table 4).
DISCUSSION
CSR seen in acute stroke is associated with the previous mRS score, bilateral lesions, a low LVEF, and LA enlargement. CSR was not observed in acute stroke due to SVO except for one patient who developed symptoms of heart failure 1 month later. There was no significant association between the location or severity of the stroke and CSR. In the subgroup analysis that excluded cardiac factors, only the previous mRS score was significantly associated with CSR.
While the associations of heart failure and decreased LVEF with CSR are well known, the present association between LA enlargement and CSR is a novel finding. LA enlargement is a sign of overloading of both the pressure and volume of the left ventricle, and an indicator of diastolic dysfunction. LA enlargement is frequently seen in patients with hypertension, and has been reported to be associated with cardiovascular diseases such as atrial fibrillation, heart failure, and stroke, as well as poor outcomes.
21 Among the indices of atrium size, LAVI has been known to have the strongest correlation with cardiovascular disease and its outcome.
22 However, there is also a tendency for the atrium to enlarge with age regardless of disease.
23 In our study, even after correcting for atrial fibrillation and decreased LVEF using multivariate analysis, there was still an independent association between CSR and an increase in LAVI. Similar results were found in a study of sleep disordered breathing in patients with heart failure but preserved LVEF, demonstrating that the size of the LA was larger in the CSA group.
24 Those authors suggested that pulmonary congestion can lead to CSA. However, in our study, there had been only one patient who had shown heart failure with preserved LVEF, which developed one month after stroke, and this makes it difficult to explain the mechanism underlying CSR on the basis of pulmonary congestion. A delay in circulation as a result of atrium enlargement or diastolic dysfunction might have caused instability in the ventilatory control system, leading to CSR.
25 Moreover, cardiovascular aging represented by LA enlargement might have been a predisposing factor for the development of CSR.
However, these results alone cannot be used to determine whether LA enlargement itself in patients with acute stroke was the predisposing factor for developing CSR or an innocent bystander that had not been included in the study analysis.
Considering that CSR manifests immediately after the onset of acute stroke, which tends to improve spontaneously with time,
259 CSR in acute stroke might not occur solely due to age or cardiac factors, but rather the stroke itself must be the inducing factor for CSR. This has prompted several researchers to attempt to identify associations between the location or severity of the lesion, and CSR, but with conflicting results. As for the association between the location of the stroke and the development of CSR, there was a report stating that CSR occurs only with bilateral cerebral hemispheric lesions, while there are other reports that these lesions are not always bilateral.
71226 In addition, CSR has been reported to occur more frequently in left-side strokes, and less frequently in cases involving the left insula or mesencephalon.
1
The present study found that significantly more cases had bilateral lesions, but with no preponderance in either the left or right hemisphere. Keeping in mind that CSR may occur more frequently in strokes occurring in the infratentorial region, which includes the respiratory center, we divided the cohort into the supra- and infratentorial regions, which revealed no difference between them. There was no significant association between CSR and the location of the stroke even when classifying and analyzing the lesion according to the vascular territory. The inconsistency of the previous results could be due to the smallness of most of the studies, and no consideration of correlations between factors or correction for confounding factors. The multivariate analysis performed in the present study showed CSR to be associated with non-SVO, bilateral cerebral involvement, and previous mRS score among the neurologic factors, but not with the location or severity of the lesion. One patient with SVO did develop CSR, but this patient had complained of dyspnea on exertion at 1 month after the onset of the stroke and had been diagnosed with heart failure with preserved LVEF, and so the CSR in this patient might have been due to cardiac rather than neurologic factors. CSR has been reported to occur even in lacunar infarcts without heart failure,
27 but the diagnostic range for lacunar infarcts was broader than in our study, where exclusion criteria such as carotid stenosis of 2 cm or larger, or less than 1.5 cm with involvement of the middle cerebral artery, anterior cerebral artery, posterior cerebral artery, vertebral artery, or basilar artery stenosis had been applied; the presence of asymptomatic patients with low LVEF was not reported.
A subgroup analysis of the LAA group including patients with an LVEF of 50% or higher who did not have atrial fibrillation—in order to exclude cardiac factors and assess solely the effects of stroke—revealed that only the previous mRS score was significantly associated with the CSR group. When reviewing the associations of LA enlargement, low LVEF, bilateral lesions, and previous mRS score with CSR, we assume that the cerebrovascular insult might have caused instability of the ventilatory control system in the condition with pre-existing cardio- or cerebrovascular dysfunction.
The limitations of this study include that the assessment for CSR with sleep apnea test could not be performed in all of the patients who had presented with acute stroke. This test is not covered by National Health Insurance in Korea, and so the extreme elderly and low-income patients were more likely to be excluded from the study. Patients with a nasogastric or endotracheal tube were also excluded. These exclusions might have reduced the proportion of patients with severe stroke.
This was the largest study to date of the factors that may cause CSR in acute stroke, and its results are invaluable since confounding factors were corrected using multivariate and subgroup analyses, which has seldom been done in previous studies. The present findings indicate that CSR occurs frequently after acute stroke regardless of the location or severity of the stroke itself, but is more common in bilateral lesions and hardly occurs after SVO. Factors associated with CSR include previous neurologic deficits, low LVEF, and LA enlargement, suggesting that the presence of underlying cardio- or cerebrovascular dysfunction contribute more to the development of CSR in acute stroke than do the stroke characteristics.
Acknowledgements
This study was supported by a research grant in 2017 from Kangwon National University (No. 520170436).
References
1. Siccoli MM, Valko PO, Hermann DM, Bassetti CL. Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. J Neurol. 2008; 255:1687–1692. PMID:
19009334.

2. Hermann DM, Siccoli M, Kirov P, Gugger M, Bassetti CL. Central periodic breathing during sleep in acute ischemic stroke. Stroke. 2007; 38:1082–1084. PMID:
17255543.

3. Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002; 58:911–916. PMID:
11914407.

4. Hui DS, Choy DK, Wong LK, Ko FW, Li TS, Woo J, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in Chinese patients with first-ever ischemic stroke. Chest. 2002; 122:852–860. PMID:
12226023.
5. Parra O, Arboix A, Bechich S, García-Eroles L, Montserrat JM, López JA, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000; 161:375–380. PMID:
10673174.

6. Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999; 22:217–223. PMID:
10201066.

7. Bassetti C, Aldrich MS, Quint D. Sleep-disordered breathing in patients with acute supra- and infratentorial strokes. A prospective study of 39 patients. Stroke. 1997; 28:1765–1772. PMID:
9303023.
8. Bassetti C, Aldrich MS, Chervin RD, Quint D. Sleep apnea in patients with transient ischemic attack and stroke: a prospective study of 59 patients. Neurology. 1996; 47:1167–1173. PMID:
8909424.
9. Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006; 37:967–972. PMID:
16543515.
10. Cheyne J. A case of apoplexy, in which the fleshy part of the heart was converted into fat. Dublin Hospital. The Dublin Hospital Reports and Communications in Medicine and Surgery. Breinigsville, PA: Nabu Press;1818. p. 216–223.
11. Rowat AM, Wardlaw JM, Dennis MS. Abnormal breathing patterns in stroke: relationship with location of acute stroke lesion and prior cerebrovascular disease. J Neurol Neurosurg Psychiatry. 2007; 78:277–279. PMID:
17060339.

12. Brown HW, Plum F. The neurologic basis of Cheyne-Stokes respiration. Am J Med. 1961; 30:849–860.

13. Parra O, Arboix A, Montserrat JM, Quintó L, Bechich S, García-Eroles L. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004; 24:267–272. PMID:
15332396.

14. Sahlin C, Sandberg O, Gustafson Y, Bucht G, Carlberg B, Stenlund H, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008; 168:297–301. PMID:
18268171.
15. Stokes W. The diseases of the heart and the aorta. Dublin: Hodges and Smith;1854.
16. Naughton MT, Kee K. Sleep apnoea in heart failure: To treat or not to treat? Respirology. 2017; 22:217–229. PMID:
27998040.

17. Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005; 171:1048–1052. PMID:
15665317.
18. Bassetti CL. Sleep and stroke. In : Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. St. Louis, MO: Elsevier/Saunders;2011. p. 993–1015.
19. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. PMID:
7678184.

20. Berry RB, Brooks R, Gamaldo CE. The AASM Manual for the Scoring of Sleep and Associated Events, Rules, Terminology and Technical Specifications, Version 2.3. Darien, IL: American Academy of Sleep Medicine;2016.
21. Cuspidi C, Rescaldani M, Sala C. Prevalence of echocardiographic left-atrial enlargement in hypertension: a systematic review of recent clinical studies. Am J Hypertens. 2013; 26:456–464. PMID:
23388831.

22. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006; 47:1018–1023. PMID:
16516087.
23. Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Döring A, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009; 54:1982–1989. PMID:
19909880.
24. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009; 11:602–608. PMID:
19468022.

25. Naughton MT. Pathophysiology and treatment of Cheyne-Stokes respiration. Thorax. 1998; 53:514–518. PMID:
9713454.

26. Lee MC, Klassen AC, Resch JA. Respiratory pattern disturbances in ischemic cerebral vascular disease. Stroke. 1974; 5:612–616. PMID:
4415587.

27. Bonnin-Vilaplana M, Arboix A, Parra O, García-Eroles L, Montserrat JM, Massons J. Sleep-related breathing disorders in acute lacunar stroke. J Neurol. 2009; 256:2036–2042. PMID:
19629569.

Table 1
Demographic and clinical characteristics according to the presence of CSR (n=182)
|
No CSR (n=147) |
CSR (n=35) |
p
|
|
Demographics |
|
|
|
|
Sex, male |
84 (57.1) |
20 (57.1) |
1.000 |
|
Sex, female |
63 (42.9) |
15 (42.9) |
|
|
Age, years |
67.2±12.6 |
76.4±7.52 |
<0.0001*
|
|
Cardiovascular risk factor |
|
|
|
|
Body mass index, kg/m2
|
24.1±3.44 |
24.5±3.95 |
0.515 |
|
Previous mRS score |
0.10±0.47 |
0.46±1.04 |
0.056 |
|
Previous stroke |
14 (9.50) |
5 (14.3) |
0.408 |
|
Hypertension |
82 (55.8) |
25 (71.4) |
0.091 |
|
Diabetes mellitus |
42 (28.6) |
12 (34.3) |
0.506 |
|
Smoking |
64 (43.5) |
9 (25.7) |
0.053 |
|
Dyslipidemia |
25 (17.0) |
3 (8.60) |
0.299 |
|
Atrial fibrillation |
14 (9.50) |
11 (31.4) |
0.001*
|
|
Heart failure |
0 (0.00) |
3 (8.60) |
0.007*
|
|
Severity |
|
|
|
|
NIHSS score on admission |
3.46±4.30 |
4.83±4.84 |
0.099 |
|
Initial vital signs, mmHg |
|
|
|
|
Systolic blood pressure |
153.4±30.0 |
157.8±30.0 |
0.444 |
|
Diastolic blood pressure |
84.9±13.4 |
86.3±12.5 |
0.566 |
|
TOAST subtype |
|
|
|
|
LAA |
71 (48.3) |
21 (60.0) |
|
|
Cardioembolism |
24 (16.3) |
10 (28.6) |
|
|
Small-vessel occlusion |
32 (21.8) |
1 (2.86) |
0.007*
|
|
Stroke of other determined etiology |
5 (3.40) |
0 (0.00) |
|
|
Stroke of undetermined etiology |
15 (10.2) |
3 (8.57) |
|
|
Vascular territory |
|
|
|
|
Internal carotid artery |
7 (4.76) |
2 (5.71) |
|
|
Middle cerebral artery |
70 (47.6) |
19 (54.3) |
|
|
Anterior cerebral artery |
1 (0.68) |
1 (2.86) |
|
|
Posterior cerebral artery |
16 (10.9) |
1 (2.86) |
|
|
Basilar artery |
18 (12.2) |
3 (8.57) |
|
|
Vertebral artery |
4 (2.72) |
1 (2.86) |
|
|
Superior cerebellar artery |
1 (0.68) |
0 (0.00) |
|
|
Anterior inferior cerebellar artery |
0 (0.00) |
0 (0.00) |
|
|
Posterior inferior cerebellar artery |
15 (10.2) |
1 (2.86) |
|
|
Border zone |
2 (1.36) |
1 (2.86) |
|
|
Multiple |
13 (8.84) |
6 (17.1) |
0.149 |
|
Laterality |
|
|
|
|
Right |
71 (48.3) |
9 (25.7) |
0.079 |
|
Left |
66 (44.9) |
18 (51.4) |
|
Both |
10 (6.80) |
8 (22.9) |
0.004*
|
|
Supra- or infratentorial |
|
|
|
|
Supratentorial |
100 (68.0) |
25 (71.4) |
0.271 |
|
Infratentorial |
41 (27.9) |
6 (17.1) |
|
Both |
6 (4.10) |
4 (11.4) |
0.102 |
|
Transthoracic echocardiography |
|
|
|
|
LA size, mm |
39.1±5.67 |
42.3±6.84 |
0.005*
|
|
LAVI, mL/m2
|
37.4±12.5 |
47.1±22.0 |
0.018*
|
|
LAVI >34 mL/m2
|
74 (51.4) |
23 (67.6) |
0.043*
|
|
LVEF, % |
65.2±5.94 |
62.2±9.69 |
0.095 |
|
LVEF <50% |
4 (2.80) |
7 (20.6) |
0.001*
|
|
Valve disease |
4 (2.80) |
1 (2.90) |
1.000 |
|
Regional wall-motion abnormality |
6 (4.20) |
4 (11.8) |
0.099 |
|
Pulmonary hypertension (borderline or more) |
10 (6.90) |
5 (14.7) |
0.143 |
|
Time to sleep study from onset, days |
8.72±9.63 |
9.43±7.49 |
0.685 |
|
Total apnea hypopnea index |
16.1±16.4 |
41.9±13.6 |
<0.001 |
|
Central apnea index |
1.33±2.38 |
14.2±8.69 |
<0.001 |
|
Obstructive apnea index |
4.89±7.79 |
8.04±10.9 |
0.113 |
|
Mixed apnea index |
1.76±5.83 |
6.51±5.59 |
<0.001 |
|
Lowest oxygen saturation |
86.8±5.53 |
84.8±5.46 |
0.060 |
Table 2
Results of multivariate analysis to identify the factors associated with Cheyne-Stokes respiration
|
OR |
95% CI |
p
|
|
Age |
1.05 |
0.991–1.12 |
0.094 |
|
Hypertension |
1.19 |
0.386–3.68 |
0.760 |
|
Smoking |
0.911 |
0.302–2.75 |
0.869 |
|
Previous mRS score |
2.32 |
1.11–4.84 |
0.025*
|
|
NIHSS score on admission |
0.959 |
0.859–1.07 |
0.453 |
|
Atrial fibrillation |
2.63 |
0.775–8.93 |
0.121 |
|
LAA or cardioembolism |
4.97 |
0.554–44.6 |
0.152 |
|
Laterality (bilateral) |
6.47 |
1.51–27.7 |
0.012*
|
|
LVEF <50% |
6.22 |
1.41–27.5 |
0.016*
|
|
LAVI (per 10 mL/m2 increase) |
1.36 |
1.00–1.85 |
0.048*
|
Table 3
Demographic and clinical characteristics according to the presence of CSR in the LAA subgroup (n=83)
|
No CSR (n=67) |
CSR (n=16) |
p
|
|
Demographics |
|
|
|
|
Sex, male |
35 (52.2) |
10 (62.5) |
0.459 |
|
Sex, female |
32 (47.8) |
6 (37.5) |
|
|
Age, years |
69.3±12.1 |
76.1±8.54 |
0.036*
|
|
Cardiovascular risk factor |
|
|
|
|
Body mass index, kg/m2
|
24.3±3.02 |
24.8±2.08 |
0.454 |
|
Previous mRS score |
0.04±0.37 |
0.69±1.35 |
0.078 |
|
Previous stroke |
4 (6.00) |
3 (18.8) |
0.127 |
|
Hypertension |
38 (56.7) |
11 (68.8) |
0.379 |
|
Diabetes mellitus |
19 (28.4) |
6 (37.5) |
0.474 |
|
Smoking |
31 (46.3) |
4 (25.0) |
0.162 |
|
Dyslipidemia |
11 (16.4) |
2 (12.5) |
1.000 |
|
Atrial fibrillation |
0 (0.00) |
0 (0.00) |
|
|
Heart failure |
0 (0.00) |
0 (0.00) |
|
|
Severity |
|
|
|
|
NIHSS score on admission |
3.31±3.92 |
4.81±5.26 |
0.203 |
|
Initial vital signs, mm Hg |
|
|
|
|
Systolic blood pressure |
158.7±30.4 |
159.0±31.8 |
0.977 |
|
Diastolic blood pressure |
84.9±15.1 |
83.8±9.06 |
0.782 |
|
Vascular territory |
|
|
|
|
Internal carotid artery |
5 (7.46) |
1 (6.25) |
|
|
Middle cerebral artery |
26 (38.8) |
7 (43.8) |
|
|
Anterior cerebral artery |
1 (1.49) |
0 (0.00) |
|
|
Posterior cerebral artery |
3 (4.48) |
0 (0.00) |
|
|
Basilar artery |
13 (19.4) |
2 (12.5) |
|
|
Vertebral artery |
1 (1.49) |
1 (6.25) |
|
|
Superior cerebellar artery |
0 (0.00) |
0 (0.00) |
|
|
Anterior inferior cerebellar artery |
0 (0.00) |
0 (0.00) |
|
|
Posterior inferior cerebellar artery |
9 (13.4) |
0 (0.00) |
|
|
Border zone |
2 (2.99) |
1 (6.25) |
|
|
Multiple |
7 (10.4) |
4 (25.0) |
0.210 |
|
Laterality |
|
|
|
|
Right |
35 (52.2) |
4 (25.0) |
0.205 |
|
Left |
26 (38.8) |
8 (50.0) |
|
Both |
6 (9.00) |
4 (25.0) |
0.095 |
|
Supra- or infratentorial |
|
|
|
|
Supratentorial |
39 (58.2) |
9 (56.3) |
0.756 |
|
Infratentorial |
26 (38.8) |
4 (25.0) |
|
Both |
2 (3.00) |
3 (18.8) |
0.047*
|
|
Transthoracic echocardiography |
|
|
|
|
LA size, mm |
38.1±4.46 |
39.5±7.21 |
0.339 |
|
LAVI, mL/m2
|
36.0±9.44 |
39.4±19.0 |
0.488 |
|
LVEF, % |
65.6±4.82 |
66.3±4.54 |
0.616 |
|
Valve disease |
0 (0.00) |
0 (0.00) |
|
|
Regional wall-motion abnormality |
0 (0.00) |
0 (0.00) |
|
|
Pulmonary hypertension (borderline or greater) |
5 (7.50) |
1 (6.30) |
1.000 |
|
Time to sleep study from onset, days |
8.70±8.73 |
9.06±5.79 |
0.876 |
|
Total apnea hypopnea index |
16.7±17.1 |
45.0±14.8 |
<0.001 |
|
Central apnea index |
1.17±2.02 |
11.9±6.67 |
<0.001 |
|
Obstructive apnea index |
5.76±8.41 |
10.8±13.5 |
0.170 |
|
Mixed apnea index |
1.64±5.72 |
7.14±6.68 |
0.005 |
|
Lowest oxygen saturation |
86.3±4.83 |
83.5±6.52 |
0.056 |
Table 4
Results of multivariate analysis to identify the factors associated with Cheyne-Stokes respiration in the large-artery atherosclerosis subgroup (n=83)
|
OR |
95% CI |
p
|
|
Age |
1.06 |
0.99–1.14 |
0.093 |
|
Previous mRS score |
2.51 |
1.04–6.08 |
0.041*
|
|
Laterality (bilateral) |
2.05 |
0.19–22.0 |
0.553 |
|
Supra- or infratentorial (both) |
3.02 |
0.15–59.9 |
0.469 |








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download