1. Headache Classification Committee of the International Headache Society (IHS).The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID:
23771276.
2. Tan CT, Mao Z, Qiu W, Hu X, Wingerchuk DM, Weinshenker BG. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2016; 86:491–492. PMID:
26833940.
3. Massiou H, Launay JM, Levy C, El Amrani M, Emperauger B, Bousser MG. SUNCT syndrome in two patients with prolactinomas and bromocriptine-induced attacks. Neurology. 2002; 58:1698–1699. PMID:
12058109.

4. Blättler T, Capone Mori A, Boltshauser E, Bassetti C. Symptomatic SUNCT in an eleven-year-old girl. Neurology. 2003; 60:2012–2013. PMID:
12821758.

5. Kaphan E, Eusebio A, Donnet A, Witjas T, Chérif A. Shortlasting, unilateral, neuralgiform headache attacks with conjunctival injection and tearing (SUNCT syndrome) and tumour of the cavernous sinus. Cephalalgia. 2003; 23:395–397. PMID:
12780771.

6. Lim EC, Teoh HL. Headache - it's more than meets the eye: orbital lesion masquerading as SUNCT. Cephalalgia. 2003; 23:558–560. PMID:
12950384.

7. Matharu MS, Levy MJ, Merry RT, Goadsby PJ. SUNCT syndrome secondary to prolactinoma. J Neurol Neurosurg Psychiatry. 2003; 74:1590–1592. PMID:
14617728.

8. Black D, Swanson J, Eross E, Cutrer FM. Secondary SUNCT due to intraorbital, metastatic bronchial carcinoid. Cephalalgia. 2005; 25:633–635. PMID:
16033391.

9. Leroux E, Schwedt TJ, Black DF, Dodick DW. Intractable SUNCT cured after resection of a pituitary microadenoma. Can J Neurol Sci. 2006; 33:411–413. PMID:
17168168.

10. Rocha Filho PA, Galvão AC, Teixeira MJ, Rabello GD, Fortini I, Calderaro M, et al. SUNCT syndrome associated with pituitary tumor: case report. Arq Neuropsiquiatr. 2006; 64:507–510. PMID:
16917628.

11. Rozen TD. Resolution of SUNCT after removal of a pituitary adenoma in mild acromegaly. Neurology. 2006; 67:724. PMID:
16924039.

12. Jiménez Caballero . SUNCT syndrome in a patient with prolactinoma and cabergoline-induced attacks. Cephalalgia. 2007; 27:76–78. PMID:
17212687.

13. de Lourdes Figuerola M, Bruera O, Pozzo MJ, Leston J. SUNCT syndrome responding absolutely to steroids in two cases with different etiologies. J Headache Pain. 2009; 10:55–57. PMID:
19020800.

14. Zidverc-Trajkovic J, Vujovic S, Sundic A, Radojicic A, Sternic N. Bilateral SUNCT-like headache in a patient with prolactinoma responsive to lamotrigine. J Headache Pain. 2009; 10:469–472. PMID:
19763771.

15. Kutschenko A, Liebetanz D. Meningioma causing gabapentin-responsive secondary SUNCT syndrome. J Headache Pain. 2010; 11:359–361. PMID:
20428918.

16. Musuka TD, Edis RH, Kermode AG. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing caused by a pituitary adenoma. J Clin Neurosci. 2013; 20:1180–1181. PMID:
23664408.

17. Rodgers SD, Marascalchi BJ, Strom RG, Huang PP. Short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing syndrome secondary to an epidermoid tumor in the cerebellopontine angle. Neurosurg Focus. 2013; 34:E1.

18. Berk T, Silberstein S. Case report: secondary SUNCT after radiation therapy--a novel presentation. Headache. 2016; 56:397–401. PMID:
26643474.

19. Bussone G, Leone M, Dalla Volta G, Strada L, Gasparotti R, Di Monda V. Short-lasting unilateral neuralgiform headache attacks with tearing and conjunctival injection: the first “symptomatic” case? Cephalalgia. 1991; 11:123–127. PMID:
1889067.

20. Morales F, Mostacero E, Marta J, Sanchez S. Vascular malformation of the cerebellopontine angle associated with “SUNCT” syndrome. Cephalalgia. 1994; 14:301–302. PMID:
7954761.

21. De Benedittis G. SUNCT syndrome associated with cavernous angioma of the brain stem. Cephalalgia. 1996; 16:503–506. PMID:
8933996.

22. Gardella L, Viruega A, Rojas H, Nagel J. A case of a patient with SUNCT syndrome treated with Jannetta procedure. Cephalalgia. 2001; 21:996–999. PMID:
11843874.

23. Penart A, Firth M, Bowen JR. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) following presumed dorsolateral brainstem infarction. Cephalalgia. 2001; 21:236–239. PMID:
11442560.

24. Köseoglu E, Karaman Y, Kücük S, Arman F. SUNCT syndrome associated with compression of trigeminal nerve. Cephalalgia. 2005; 25:473–475. PMID:
15910575.

25. Lagares A, Gómez PA, Pérez-Nuñez A, Lobato RD, Ramos A. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing syndrome treated with microvascular decompression of the trigeminal nerve: case report. Neurosurgery. 2005; 56:E413. PMID:
15670392.

26. Sprenger T, Valet M, Platzer S, Pfaffenrath V, Steude U, Tolle TR. SUNCT: bilateral hypothalamic activation during headache attacks and resolving of symptoms after trigeminal decompression. Pain. 2005; 113:422–426. PMID:
15661452.

27. Zidverc-Trajkovic J, Mijajlovic M, Pavlovic AM, Jovanovic Z, Sternic N. Vertebral artery vascular loop in SUNCT and concomitant trigeminal neuralgia. case report. Cephalalgia. 2005; 25:554–557. PMID:
15955046.

28. Mondéjar B, Cano EF, Pérez I, Navarro S, Garrido JA, Velásquez JM, et al. Secondary SUNCT syndrome to a variant of the vertebrobasilar vascular development. Cephalalgia. 2006; 26:620–622. PMID:
16674773.

29. Rodrigues GG, Bordini CA, Dach F, Eckeli A, Speciali JG. SUNCT syndrome: report of a possible symptomatic case. Arq Neuropsiquiatr. 2007; 65:852–854. PMID:
17952296.

30. Guerreiro R, Casimiro M, Lopes D, Marques JP, Fontoura P. Video NeuroImage: symptomatic SUNCT syndrome cured after trigeminal neurovascular contact surgical decompression. Neurology. 2009; 72:e37. PMID:
19221289.

31. Irimia P, González-Redondo R, Domínguez PD, Díez-Valle R, Martínez-Vila E. Microvascular decompression may be effective for refractory SUNCT regardless of symptom duration. Cephalalgia. 2010; 30:626–630. PMID:
19614700.

32. Williams M, Bazina R, Tan L, Rice H, Broadley SA. Microvascular decompression of the trigeminal nerve in the treatment of SUNCT and SUNA. J Neurol Neurosurg Psychiatry. 2010; 81:992–996. PMID:
20462914.

33. Bartsch T, Falk D, Knudsen K, Reese R, Raethjen J, Mehdorn HM, et al. Deep brain stimulation of the posterior hypothalamic area in intractable short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT). Cephalalgia. 2011; 31:1405–1408. PMID:
21628443.

34. Chaila E, Ali E, Rawluk D, Hutchinson M. ‘Switching off’ SUNCT by sudden head movement: a new symptom. J Neurol. 2011; 258:694–695. PMID:
21072534.

35. Paliwal VK, Singh P, Kumar A, Rahi SK, Gupta RK. Short-lasting unilateral neuralgiform headache with conjunctival injection and tearing (SUNCT) with preserved refractory period: report of three cases. J Headache Pain. 2012; 13:167–169. PMID:
22227716.

36. Favoni V, Grimaldi D, Pierangeli G, Cortelli P, Cevoli S. SUNCT/ SUNA and neurovascular compression: new cases and critical literature review. Cephalalgia. 2013; 33:1337–1348. PMID:
23800827.
37. Jin D, Lian YJ, Zhang HF. Secondary SUNCT syndrome caused by dorsolateral medullary infarction. J Headache Pain. 2016; 17:12. PMID:
26885826.

38. Lambru G, Trimboli M, Tan SV, Al-Kaisy A. Medullary infarction causing coexistent SUNCT and trigeminal neuralgia. Cephalalgia. 2017; 37:486–490. PMID:
27226002.

39. Kitahara I, Fukuda A, Imamura Y, Ikawa M, Yokochi T. Pathogenesis, surgical treatment, and cure for SUNCT syndrome. World Neurosurg. 2015; 84:1080–1083. PMID:
26008143.

40. Williams MH, Broadley SA. SUNCT and SUNA: clinical features and medical treatment. J Clin Neurosci. 2008; 15:526–534. PMID:
18325769.

41. Coskun O, Ucar M, Vuralli D, Yildirim F, Cetinkaya R, Akin Takmaz S, et al. MR tractography in short lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT) patients: case reports. Pain Med. 2017; 18:1377–1381. PMID:
28339630.

42. Ertsey C, Bozsik G, Afra J, Jelencsik I. A case of SUNCT syndrome with neurovascular compression. Cephalalgia. 2000; 20:325.
43. Maggioni F, Manara R, Mampreso E, Viaro F, Mainardi F, Zanchin G. Trigeminal neuralgia and trigeminal-autonomic cephalalgias: a continuum or simple co-existence? Cephalalgia. 2010; 30:752–756. PMID:
19624686.

44. Putzki N, Nirkko A, Diener HC. Trigeminal autonomic cephalalgias: a case of post-traumatic SUNCT syndrome? Cephalalgia. 2005; 25:395–397. PMID:
15839855.

45. Choi HJ, Choi SK, Lee SH, Lim YJ. Whiplash injury-induced atypical short-lasting unilateral neuralgiform headache with conjunctival injection and tearing syndrome treated by greater occipital nerve block. Clin J Pain. 2012; 28:342–343. PMID:
22330130.

46. Hannerz J, Greitz D, Hansson P, Ericson K. SUNCT may be another manifestation of orbital venous vasculitis. Headache. 1992; 32:384–389. PMID:
1399559.

47. Bichuetti DB, Yamaoka WY, Bastos JR, Carvalho Dde S. Bilateral SUNCT syndrome associated to chronic maxillary sinus disease. Arq Neuropsiquiatr. 2006; 64:504–506. PMID:
16917627.

48. Eguia P, Garcia-Monco JC, Ruiz-Lavilla N, Diaz-Konrad V, Monton F. SUNCT and trigeminal neuralgia attributed to meningoencephalitis. J Headache Pain. 2008; 9:51–53. PMID:
18217200.

49. Choi JY, Seo WK, Kim JH, Oh K, Yu SW. Symptomatic SUNCT syndrome associated with ipsilateral paranasal sinusitis. Headache. 2008; 48:1527–1530. PMID:
19076651.

50. Ito Y, Yamamoto T, Ninomiya M, Mizoi Y, Itokawa K, Tamura N, et al. Secondary SUNCT syndrome caused by viral meningitis. J Neurol. 2009; 256:667–668. PMID:
19444540.

51. Cascella C, Rosen JB, Robbins MS, Levin M. Resident and fellow section. teaching case: symptomatic SUNCT. Headache. 2011; 51:1022–1026. PMID:
21631490.
52. Pong DL, Marom T, Pine HS. Short-lasting unilateral neuralgiform headache attacks with conjunctiva injection and tearing presenting as sphenoiditis. Am J Otolaryngol. 2013; 34:166–168. PMID:
23201466.

53. Granato A, Belluzzo M, Fantini J, Zorzon M, Koscica N. SUNCT-like syndrome attributed to varicella-zoster virus meningoencephalitis. Neurol Sci. 2015; 36:807–808. PMID:
25073697.

54. Mathew T, Srinivas M, Badachi S, Nadig R. Post herpes zoster SUNCT like syndrome: insights from two case reports. Cephalalgia. 2018; 38:399–401. PMID:
27799443.

55. Kursun O, Arsava EM, Oguz KK, Tan E, Kansu T. SUNCT associated with Devic's syndrome. Cephalalgia. 2006; 26:221–224. PMID:
16426279.

56. Vilisaar J, Constantinescu CS. SUNCT in multiple sclerosis. Cephalalgia. 2006; 26:891–893. PMID:
16776709.

57. Bogorad I, Blum S, Green M. A case of MS presenting with SUNCT status. Headache. 2010; 50:141–143. PMID:
19751367.

58. ter Berg JW, Goadsby PJ. Significance of atypical presentation of symptomatic SUNCT: a case report. J Neurol Neurosurg Psychiatry. 2001; 70:244–246. PMID:
11160478.

59. Morís G, Ribacoba R, Solar DN, Vidal JA. SUNCT syndrome and seborrheic dermatitis associated with craneosynostosis. Cephalalgia. 2001; 21:157–159. PMID:
11422101.

60. Sebastian S, Schweitzer D, Tan L, Broadley SA. Role of trigeminal microvascular decompression in the treatment of SUNCT and SUNA. Curr Pain Headache Rep. 2013; 17:332. PMID:
23564233.

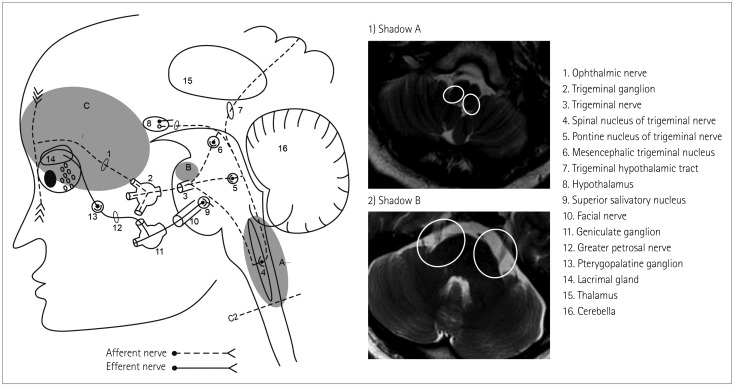
61. Zhang X. Trigeminohypothalamic tract. In : Gebhart GF, Schmidt RF, editors. Encyclopedia of Pain. 2nd ed. Berlin: Springer-Verlag Berlin Heidelberg;2013. p. 4082.
62. Kao YH, Hsu YC. Chiari malformation type I presenting as clusterlike headache. Acta Neurol Taiwan. 2015; 24:122–124. PMID:
27333966.
63. Kim DE, Park JY, Ha H, Yoon GJ, Cho BH, Lee SH. Cluster-like headache secondary to focal cervical myelitis. Neurologist. 2017; 22:138–140. PMID:
28644256.

64. Budhram A, Leung A, Sangle N, Khaw AV. Diffuse large B-cell lymphoma of the nasopharynx presenting with cluster-like headache. Headache. 2017; 57:806–808. PMID:
28295284.

65. Kuritzky A. Cluster headache-like pain caused by an upper cervical meningioma. Cephalalgia. 1984; 4:185–186. PMID:
6498932.

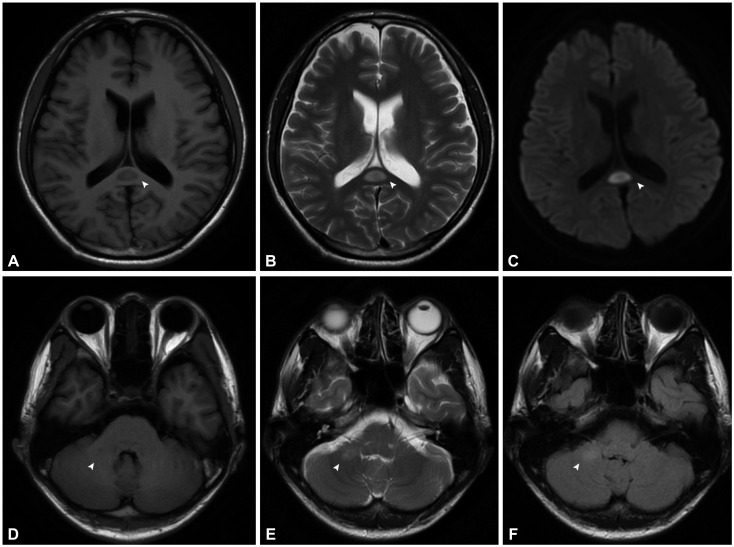
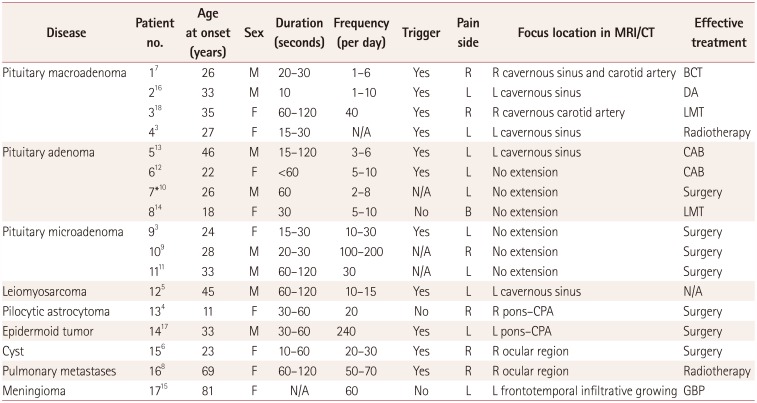
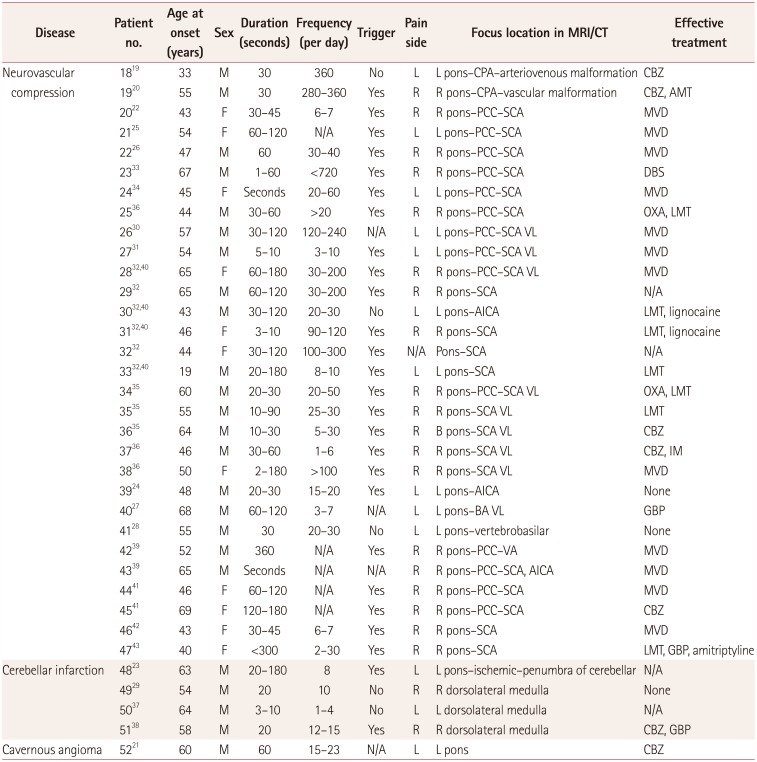




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download