Abstract
Cell permeable peptide (CPP) is able to transport itself or conjugated molecules such as nucleotides, peptides, and proteins into cells. Since short peptide of human immunodeficiency virus-1 Tat has been discovered as CPP, it has been continuously studied for their ability to transport heterologous cargoes into cells. In this study, we have focused on the fusion protein of respiratory syncytial virus (RSV), which has six basic amino acids in multi basic furin-dependent cleavage site (MBFCS) required to be cationic CPP. To develop more efficient CPP, the sequence, which linked two MBFCS, was synthesized (called RS-CPP). To assess cell permeable efficiency of RS-CPP or MBFCS, the peptides was conjugated with fluorescein isothiocyanate, and cell permeable efficiency was measured by fluorescence-activated cell sorting. Cell permeability of RS-CPP or MBFCS was increased in a dose-dependent manner, but RS-CPP showed more efficient cell permeability than MBFCS in MDCK, HeLa, Vero E6, and A549 cells. To evaluate whether RS-CPP can transport its conjugated functional peptide (VIVIT) in CD8+ T cell, it was confirmed that IL-2 and β-galactosidase expression were significantly inhibited through selective block of nuclear factor activated T-cell. To investigate endocytic pathways, Cre-mediated DNA recombination (loxP-STOP-loxP-LacZ reporter system) was investigated with divergent endocytosis inhibitors in TE671 cells, and RS-CPP endocytosis is occurred via binding cell surface glycosaminoglycan and clathrin-mediated endocytosis, or macropinocytosis. These results indicated that RS-CPP could be a novel cationic CPP, and it would help understanding for delivery of biologically functional molecules based on viral basic amino acids.
Figures and Tables
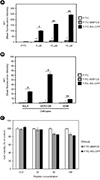 | Figure 1Cell permeability of MBFCS and RS-CPP in various cells. (A) Cell permeability of FITC-MBFCS or FITC-RS-CPP in MDCK cells. MDCK cells were treated with indicated concentration of FITC-MBFCS (gray bar) or FITC-RS-CPP (black bar) for 30 m. After incubation, the cells were washed three times with PBS, and mean fluorescence intensity (MFI) was measured by FACS. (B) Cell permeability of FITC-MBFCS or FITC-RS-CPP in HeLa, VERO-E6, and A549 cells. 15 µM of FITC-MBFCS or FITC-RS-CPP were treated in HeLa, VERO-E6, and A549 cells, respectively, for 30 m, and the cells were washed three times with PBS. MFI was measured by FACS. The MFI of FITC (open bar) is individually set to 1, and the rest were plotted relative to that value. Error bars represent ± SD. Statically significant different values between FITC and FITC-MBFCS represented as *p < 0.05 and **p < 0.01 and between FITC-MBFCS and FITC-RS-CPP as #p < 0.05 and ##p < 0.01. (C) Cell viability measurement in MDCK cells. MDCK cells were treated with indicated concentrations (serial diluted from 100 µM to 12.5 µM) of FITC-MBFCS or FITC-RS-CPP, respectively, and incubated for 30 m. Cell viability was measured using an Ez-cytox kit, according to manufacturer's protocol. The O.D. value of mock was set to 100% and all other values were plotted as relative values. |
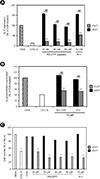 | Figure 2Cell permeability of RS-CPP with VIVIT peptide. Expression of (A) IL-2 or (B) β-galactosidase by the treatment of indicated concentration of RS-CPP or R11 conjugated with VIVIT or VEET peptide. Co-cultured B3Z (CD8+ T cells bearing OVA-peptide specific TCR stimulation) and JAWSII (syngenic antigen presenting cell for B3Z) cells were treated with indicated concentration of RS-CPP-VIVIT (gray bars) or -VEET (black bars) peptide, respectively, for 30 m. Subsequently, the cells were incubated for 12 h under presence of 5 µM OVA-peptide (stripe bar), and expression level of IL-2 or β-galactosidase was measured by optical density. Cyclosporin A (CYC A, open bar) was used as an inhibitor of T cell activation, and OVA-peptide was used as an activator of NFAT in B3Z T cell. R11 was used as a cell penetrating vehicle. Error bars represent ± SD. The IL-2 or β-galactosidase expression level of OVA was set to 100%, respectively, and the rest were plotted relative to that value. Error bars represent ± SD. Statically significant different values between OVA and RS-CPP-VIVIT represented as **p < 0.01 and between RS-CPP-VEER and RS-CPP-VIVIT as ##p < 0.01. (C) Cell viability measurement with RS-CPP-VIVIT and -VEET peptides in Co-cultured B3Z and JAWSII cells. The co-cultured cells were treated with indicated concentrations of RS-CPP-VIVIT, RS-CPP-VEET, R11-VIVIT, and R11-VEET peptide, respectively, and incubated for 30 m. Cell viability was measured using a cell viability test kit (Ez-cytox), according to manufacturer's protocol. The O.D. value of mock was set to 100% and all other values were plotted as relative values. Statically significant different values between Mock and RS-CPP-VIVIT **p < 0.01 Asterisk represented statistically significantly differences compared with mock (*p < 0.05). |
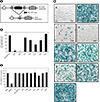 | Figure 3RS-CPP mediated DNA recombination. (A) Schematic illustration of loxP.STOP.loxP.LacZ expression system in TE671 cells. Internalization of RS-CPP-Cre (DNA recombinase) eliminates the STOP region flanked by loxP sites, resulting in LacZ expression in TE671 cells. (B) Light microscopy images of LacZ expression in TE671 cells by treating (a) 2 µM of Cre, (b) RS-CPP-Cre, or (c-i) RS-CPP-Cre with indicated endocytic inhibitors. TE671 cells were pretreated with indicated inhibitors for 30 m and incubated under presence of 2 µM of RS-CPP-Cre for 48 h. Subsequently, LacZ expression was examined by X-gal staining under light microscope. Scale bar represents 20 µM. (C) Relative % values of LacZ expression cells between RS-CPP-Cre and the other inhibitor treated cells. LacZ expression cells of RS-CPP-Cre were set to 100%, and the rests were shown as relative value. The cells were counted from three different fields of each sample (Magnification; 200×). Values significantly different from RS-CPP-Cre were represented as *p < 0.05 and **p < 0.01. (D) Cell viability measurement with RS-CPP-Cre and endocytic inhibitors in TE671 cells. TE671 cells were treated with 2 µM of Cre and RS-CPP-Cre, or RS-CPP-Cre with indicated endocytic inhibitors, respectively, for 30 m (AML and CPZ for 15 m). 5 µM of melittin was used as a cytotoxicity control. Cell viability was measured a cell viability test kit (Ez-cytox), according to manufacturer's protocol. The O.D. value of mock was set to 100% and all other values were plotted as relative values. Statically significant different values between control and the endocytic inhibitors were represented as *p < 0.05. |
ACKNOWLEDGEMENT
This research was supported by intramural grants from the Korea National Institute of Health 2011-N43001-00 and 2016-NI43001-00.
References
1. Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009; 157:195–206.

2. Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012; 17:850–860.

3. Mäe M, Langel Ü. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006; 6:509–514.

4. Rizzuti M, Nizzardo M, Zanetta C, Ramirez A, Corti S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov Today. 2015; 20:76–85.

5. Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004; 10:310–315.

6. Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997; 272:16010–16017.

7. Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004; 6:189–196.

8. Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001; 276:5836–5840.
9. Hsieh JT, Zhou J, Gore C, Zimmern P. R11, a novel cell-permeable peptide, as an intravesical delivery vehicle. BJU Int. 2011; 108:1666–1671.

10. Tünnemann G, Ter-Avetisyan G, Martin RM, Stöckl M, Herrmann A, Cardoso MC. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J Pept Sci. 2008; 14:469–476.

11. McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013; 372:83–104.

12. González-Reyes L, Ruiz-Argüello MB, García-Barreno B, Calder L, López JA, Albar JP, et al. Cleavage of the human respiratory syncytial virus fusion protein at two distinct sites is required for activation of membrane fusion. Proc Natl Acad Sci U S A. 2001; 98:9859–9864.

13. Klimstra WB, Heidner HW, Johnston RE. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J Virol. 1999; 73:6299–6306.

14. Rawling J, Cano O, Garcin D, Kolakofsky D, Melero JA. Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus. J Virol. 2011; 85:2771–2780.

15. Noguchi H, Matsushita M, Okitsu T, Moriwaki A, Tomizawa K, Kang S, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004; 10:305–309.

16. Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci U S A. 1992; 89:6020–6024.

17. Fiering S, Northrop JP, Nolan GP, Mattila PS, Crabtree GR, Herzenberg LA. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990; 4:1823–1834.

18. Chow CW, Rincón M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol Cell Biol. 1999; 19:2300–2307.

19. Xu Y, Liu S, Yu G, Chen J, Chen J, Xu X, et al. Excision of selectable genes from transgenic goat cells by a protein transducible TAT-Cre recombinase. Gene. 2008; 419:70–74.

20. Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc Natl Acad Sci U S A. 2002; 99:4489–4494.

21. Montrose K, Yang Y, Sun X, Wiles S, Krissansen GW. Xentry, a new class of cell-penetrating peptide uniquely equipped for delivery of drugs. Sci Rep. 2013; 3:1661.

22. Fang SL, Fan TC, Fu HW, Chen CJ, Hwang CS, Hung TJ, et al. A novel cell-penetrating peptide derived from human eosinophil cationic protein. PLoS One. 2013; 8:e57318.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download