Abstract
Acute generalized exanthematous pustulosis (AGEP) is rarely caused by radiocontrast media (RCM). The role of skin tests for the diagnosis and evaluation of cross-reactivity in a delayed type of RCM-induced hypersensitivity have yet to be determined. Here, we report a case of iodixanol-induced AGEP where we safely administered alternative RCM using patch tests. A 44-year-old woman had coronary artery angiography (CAG) for the evaluation of ischemic heart disease. She was on regular hemodialysis because of end-stage renal disease. She was given iodixanol (Visipaque) during CAG. Approximately 1 day after CAG, she developed AGEP. The patient was rehospitalized for CAG again after 1 year. We performed skin tests to choose safe alternative RCM. Intradermal tests with iodixanol, iohexol (Bonorex) and Iopamidol (Pamiray) showed negative responses. Patch tests showed a positive response to iodixanol, equivocal to iohexol, and negative to Iopamidol. We finally chose Iopamidol and performed CAG successfully without any adverse reaction. Patch tests may be a useful tool for the diagnosis and choice of safe alternatives in RCM-induced delayed-type hypersensitivity reactions such as AGEP.
Figures and Tables
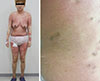
Fig. 1
Generalized exanthematous rash with numerous small pustules was shown 1 day after coronary angiography using iodixanol (Visipaque, GE Healthcare, Chicago, IL, USA).
References
1. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001; 28:113–119.

2. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007; 157:989–996.

3. Poliak N, Elias M, Cianferoni A, Treat J. Acute generalized exanthematous pustulosis: the first pediatric case caused by a contrast agent. Ann Allergy Asthma Immunol. 2010; 105:242–243.

4. Bavbek S, Sözener ZC, Aydin O, Ozdemir SK, Gül U, Heper AO. First case report of acute generalized exanthematous pustulosis due to intravenous iopromide. J Investig Allergol Clin Immunol. 2014; 24:66–67.
5. Peterson A, Katzberg RW, Fung MA, Wootton-Gorges SL, Dager W. Acute generalized exanthematous pustulosis as a delayed dermatotoxic reaction to IV-administered nonionic contrast media. AJR Am J Roentgenol. 2006; 187:W198–W201.

6. Atasoy M, Erdem T, Sari RA. A case of acute generalized exanthematous pustulosis (AGEP) possibly induced by iohexol. J Dermatol. 2003; 30:723–726.

7. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009; 145:683–687.

8. Grandvuillemin A, Ripert C, Sgro C, Collet E. Iodinated contrast media-induced acute generalized exanthematous pustulosis confirmed by delayed skin tests. J Allergy Clin Immunol Pract. 2014; 2:805–806.

9. Kim SJ, Lee T, Lee YS, Bae YJ, Cho YS, Moon HB, et al. Acute generalized exanthematous pustulosis caused by radiocontrast media. Ann Allergy Asthma Immunol. 2010; 105:492–493.

10. Brockow K. Immediate and delayed cutaneous reactions to radiocontrast media. Chem Immunol Allergy. 2012; 97:180–190.

11. ACR Manual on contrast media, v 10.3. ACR Committee on Drugs and Contrast Media [Internet]. Reston (VA): American College of Radiology;2017. cited 2016 Dec 7. Available from: https://www.braccoimaging.com/sites/braccoimaging.com/files/technica_sheet_pdf/us-en-2018-01-26-manual-ACR-Contrast-Media.pdf.
12. ESUR guidelines on contrasts media, v 8.1 [Internet]. Wien (Austria): European Society of Urogenital Radiology;2014. cited 2016 Dec 7. Available from: https://www.esur.org/esurguidelines/contrast-media-81/.
13. Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy. 2009; 64:234–241.

14. Yoon SH, Lee SY, Kang HR, Kim JY, Hahn S, Park CM, et al. Skin tests in patients with hypersensitivity reaction to iodinated contrast media: a meta-analysis. Allergy. 2015; 70:625–637.

15. Caro JJ, Trindade E, McGregor M. The risks of death and of severe nonfatal reactions with high- vs low-osmolality contrast media: a meta-analysis. AJR Am J Roentgenol. 1991; 156:825–832.

16. Gomi T, Nagamoto M, Hasegawa M, Katoh A, Sugiyama M, Murata N, et al. Are there any differences in acute adverse reactions among five low-osmolar non-ionic iodinated contrast media? Eur Radiol. 2010; 20:1631–1635.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download