Abstract
A 38-year-old man, who underwent a second kidney transplantation (KT), was admitted because of antibody-mediated rejection (AMR) complicated by BK virus-associated nephropathy (BKVAN). He was placed on hemodialysis at the age of 24 years because of membranoproliferative glomerulonephritis. At the age of 28 years, he underwent a living donor KT from his father; however, 1 year after the transplantation, he developed a recurrence of the primary glomerular disease, resulting in graft failure 2 years after the first KT. Ten years later, he received a deceased-donor kidney with a B-cell-positive-cross-match. He received 600 mg of rituximab before the KT with three cycles of plasmapheresis and immunoglobulin (0.5 g/kg) therapy after KT. During the follow-up, the first and second allograft biopsies at 4 and 10 months after KT revealed AMR with a recurrence of primary glomerular disease that was reclassified as C3 glomerulonephritis (C3GN). He received a steroid pulse, rituximab, plasmapheresis, and immunoglobulin therapies. The third allograft biopsy demonstrated that the BKVAN was complicated with AMR and C3GN. As the azotemia did not improve after repeated conventional therapies for AMR, one cycle of bortezomib (1.3 mg/m2×4 doses) was administered. The allograft function stabilized, and BK viremia became undetectable after 6 months. The present case suggests that bortezomib therapy may be applicable to patients with refractory AMR, even in cases complicated with BKVAN.
Antibody-mediated rejection (AMR) is one of the most common causes of allograft dysfunction and allograft failure in kidney transplant recipients (KTRs) because it is often unresponsive to the current conventional treatments(1). The Kidney Disease: Improving Global Outcomes recommendations recognize that no definitive treatment is available for AMR and that steroids, plasma exchange, intravenous immunoglobulin (IVIG), anti-CD20 monoclonal antibodies, and T-cell depleting agents have all been used with some success(2). Although some strategies for AMR treatment have been studied in controlled clinical trials, no treatments are currently recommended by the international guidelines(345). Thus, AMR remains a therapeutic challenge for physicians.
The conventional treatments for AMR have focused on antibody removal and B-cell depletion; however, these treatments do not target the plasma cells that produce the antibodies. Recently, bortezomib, a proteasome inhibitor, has been used as a plasma cell-targeting agent during transplantation(67) and has been shown to reduce antibodies by depleting antibody-producing plasma cells and blocking anti-human leukocyte antigen (HLA) immunoglobulin G secretion. In previous studies, the use of bortezomib for the treatment of refractory AMR and primary treatment of AMR has also been reported(48910). These previous case series using bortezomib therapy as a treatment for refractory and primary AMR show that bortezomib can be an alternative treatment for such recipients.
Advanced immunosuppressive agents have reduced acute rejection rates in KTRs. However, some infectious diseases caused by more intensive immunosuppression could lead to complications and make the choice of treatment more difficult. BK virus-associated nephropathy (BKVAN) is one of the most important causes of allograft loss in KTRs. BKVAN still remains a major challenge in KTRs due to the high percentage of allograft loss and the paucity of effective therapeutic agents. In fact, poor clinical outcomes have been reported in several studies when AMR is associated with BKVAN(11). The incidence of BKVAN associated with acute allograft rejection was reported as 1% to 24%(1213). However, no reports have indicated that bortezomib could be used for AMR complicated with BKVAN.
Herein, we report the use of bortezomib for a patient who developed both AMR and BKVAN in the second kidney transplantation (KT), with recurrent glomerular disease.
A 38-year-old man with allograft dysfunction after a second KT was admitted to our hospital. He was initially diagnosed as having membranoproliferative glomerulonephritis (MPGN) on the basis of a renal biopsy finding in 1996. We reviewed only his pathology report, which described cellular proliferation and the lobular accentuation of the glomeruli and electron dense deposits at the mesangium and subendothelium. Immunofluorescence (IF) findings were positive only for C3 antibody and positivity for anti-immunoglobulins. He was commenced on hemodialysis because of end-stage renal disease in 2000. He received a living-donor KT from his father in 2004 and was diagnosed as having recurrence of the primary disease at 1 year after the first KT. Allograft failure developed 2 years after the first KT, and he received a B-cell cross-match-positive deceased KT in 2014. Flow cytometry cross-matching result was positive for B-cells. There were HLA-AB 2 and HLA-DR 1 mismatches and donor-specific anti-HLA antibodies (DSA) against DQ7 (mean fluorescence intensity [MFI], 19899). Considering the presence of DSA, he received desensitization therapy. He received 600 mg of rituximab before transplantation, and three cycles of plasmapheresis and immunoglobulin therapy (0.5 g/kg) postoperatively. The desensitization therapy decreased the MFI of DQ7 DSA to 3199. He also received induction therapy with basiliximab and maintenance immunosuppressive therapy with methylprednisolone, tacrolimus, and mycophenolate mofetil. The graft showed good function with a decrease in serum creatinine level and a high urinary output. valacyclovir, cotrimoxazole, and nystatin were administered for infection prophylaxis. His serum creatinine level was 1.2 mg/dL at discharge.
During follow-up, we performed allograft biopsy at 4 months after KT, as his allograft function had decreased. His serum creatinine level increased to 1.52 mg/dL without any clinical symptoms. The biopsy examination revealed microvascular inflammation (g3, ptc1, and C4d0) with recurrence of the primary disease that was reclassified as C3 glomerulonephritis (C3GN). He was also diagnosed as having AMR with recurrence of the primary disease and received steroid pulse (methylprednisolone 250 mg every 12 hours for 3 days), 600 mg of rituximab, four times of plasmapheresis, and IVIG therapy (0.5 g/kg).
The second biopsy at 10 months after KT showed sustained microvascular inflammation and C3GN (Fig. 1). Thereafter, steroid pulse (methylprednisolone 250 mg every 12 hours for 3 days), and IVIG therapy (0.5 g/kg) were repeated once. Cyclophosphamide was added for the treatment of C3GN.
The third biopsy at 11 months after KT (Fig. 2) demonstrated that stage 1 BKVAN was complicated with the previous diagnoses. Glomerular changes considerably pro gressed, and cellular crescents developed. When a polymerase chain reaction (PCR) assay for BK virus DNA in plasma was performed, the BK virus PCR titer was 10,000 copies/mL, and the tacrolimus dose was adjusted 3.5 to 5 ng/mL. When the diagnosis of BKVAN was made, a reduction of immunosuppressive agents was initiated by discontinuing the tacrolimus therapy. As the creatinine level was increased to 3.58 mg/dL even after repeated administration of the conventional therapies for AMR. One cycle of bortezomib (1.3 mg/m2×4 doses for 2 weeks) was injected. Methylprednisolone and sirolimus were used as maintenance immunosuppressants. Thereafter, allograft function was stabilized and BK viremia became undetectable after 6 months. At present, the patient has been followed up carefully at our outpatient clinic, his serum creatinine level at the latest follow-up was 3.4 mg/dL (Fig. 3).
With the progress of immunosuppressive agents and improved graft survival rates after KT, indications for KT have been further expanded(14). Thus, more high-risk recipients are receiving KT. These high-risk recipients include patients with second transplantation, multiple transfusions, long-term hemodialysis, and pregnancy(15), and these conditions can sensitize recipients and induce the production of DSA. Thus, this highly sensitized KTRs are a high-risk group for AMR. AMR is one of the most common causes of allograft failure in highly sensitized KTRs(16). Generally, AMR is treated with steroids, plasma exchange, IVIG, and rituximab. However, the efficacy of these conventional treatments is limited and has not been confirmed(17). Unlike previous studies, recent studies found that rituximab therapy has no additional benefit to graft survival(18). The efficacy of newer agents such as bortezomib and complement inhibitors in the treatment of AMR is still under research. Thus, plasma exchange and IVIG therapy have become standard treatments for AMR therapy, even though their efficacies are uncertain(19). Despite the advances in the understanding of AMR mechanisms, some aspects are still, including the role of B-cells and plasma cells.
The limitations of the conventional treatments for AMR may be that they have no direct effect on plasma cells. As a selective proteasome inhibitor, bortezomib can cause plasma cell apoptosis in the bone marrow with subsequent inhibition of antibody production and treat AMR effectively. Several transplant centers have used bortezomib to treat AMR with various success rates(792021). In these successful cases, treatment with bortezomib therapy showed reversal of graft rejection, improved renal allograft function, and decreased DSA levels. Bortezomib therapy might be an effective treatment to target plasma cells. In a recently published randomized, placebo-controlled trial, bortezomib failed to prevent glomerular filtration rate loss, improve histological or molecular disease features, or reduce DSA level. However, this randomized trial compared the effect of a single course of bortezomib treatment. Some authors have proposed that the effect of bortezomib could be enhanced by combination therapy(192223). Therefore, the effect of bortezomib for the treatment for chronic AMR should be determined after the ongoing trial to determine its efficacy, in association with steroids, plasma exchange, and polyclonal IVIG therapy.
High-intensity immunosuppression, especially after treatment of AMR, may increase the risk of infection by weakening the immune functions against pathogens. BKVAN remains a major cause of infectious diseases that can lead to allograft failure in KT(24). The cause of BKVAN is well known to be related to excessive immunosuppression(2526). Therapeutic reduction of maintenance immunosuppression can restore immunity, control viral replication, and prevent progression of BKVAN. However, the therapeutic reduction of immunosuppression can increase the risk of developing de novo DSA and acute rejection episodes and chronic AMR(27). BKVAN was considered as a complication that resulted in poor graft survival.
As in our case, BKVAN occasionally develops concurrently with acute rejection. The incidence of BKVAN associated with acute allograft rejection was reported to range from 1% to 24%(1213). Generally, acute rejection is considered to be treated first. Once the acute rejection has subsided, it is advisable to reduce the dose of immunosuppressive agents to prevent the progression of BKVAN(1228). Bortezomib therapy has been used to treat AMR in transplant recipients, and a case of BKVAN disease with plasma cell-rich infiltrates treated with bortezomib has been reported(2930). In such case, the therapeutic effect is thought to be mediated through the reduction of plasma cells and CD20+ cells. BK virus replication remained stable during the follow-up periods(29). Therefore, we speculate that bortezomib may be beneficial for refractory AMR with BKVAN because it does not activate BK virus replication. No histological examination was performed during the follow-up period, but the BK virus PCR titer was maintained at <100 copies/mL. It may indicate that bortezomib administration did not activate BK virus replication in the KTRs.
In this report, we describe our experience with bortezomib therapy for the salvage treatment of a patient who developed AMR combined with BKVAN, which was refractory to a combination of plasmaphereses with IVIG, steroid pulse, and rituximab therapy. In conclusion, our report suggests that bortezomib represents a rescue therapy for AMR refractory to conventional treatments, even in cases with concurrent BKVAN. However, during the follow-up period, the C3 level was consistently low. Approximately 15% of death-censored graft failures are due to recurrent glomerulonephritis(31). Recent data show that monoclonal- related type I MPGN has an early recurrence rate of 60%, and polyclonal-Ig-related MPGN recurrence is more variable. Low C3 and C4 levels could predict a higher risk of recurrence(3132). C3GN is the result of dysregulation in the alternative pathway of complement and is characterized by dominant staining for complement factor C3 with minimal or no staining for immunoglobulins on IF microscopy(33). C3GN recurs in 66.7% of cases with a high risk of early graft loss(34). However, no treatment has been established for C3GN, neither for the prevention of its recurrence(32). Although bortezomib may be effective in the treatment of transient rejection, it is unlikely to be effective for inhibiting C3GN progression. Future prognosis depends on the clinical effort to prevent rejection and the progression of unresolved C3GN.
Considering the limitations of our short-term follow-up period and limited experience, larger, well-designed, randomized controlled trials are essential to confirm these results and ensure the long-term clinical efficacy of bortezomib therapy for graft survival. Moreover, further evaluation is required regarding the safety and efficacy profiles of bortezomib, including its dosage and timing of administration.
Figures and Tables
Fig. 1
The second biopsy showing peritubular capillaritis (A, Periodic acid–Schiff, ×400) and focal proliferative glomerulitis with leukocytes infiltration. Only C3 is positive at the glomerular mesangium and capillary wall (B, immunofluorescence, ×200). Electron dense deposits with humplike appearance can be observed at the mesangium and subepithelium (C, electron microscopy, ×5,000). Leukocytes can be seen in the capillary lumen.
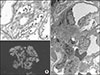
Fig. 2
The third biopsy showing proliferative glomerulitis with crescent formation. The interstitium is infiltrated by a large amount of lymphocytes (Periodic acid–Schiff, ×200). The immunohistochemical staining for BK virus shows SV 40 antibody-positive enlarged tubular epithelial cells (inset, ×200).
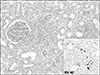
Fig. 3
Hospital progression. As the creatinine level of the patient increased to 3.58 mg/dL after repeated conventional treatments, he received one cycle of bortezomib injection (1.3 mg/m2×4 doses). Thereafter, the allograft function stabilized for 6 months. Abbreviations: PP, plasma pheresis; IVIG, intravenous immunoglobulin; Bx., biopsy; POD, post-operation day.

ACKNOWLEDGEMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HC15C1129).
References
1. Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–399.

2. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009; 9:Suppl 3. S1–155.
3. Archdeacon P, Chan M, Neuland C, Velidedeoglu E, Meyer J, Tracy L, et al. Summary of FDA antibody-mediated rejection workshop. Am J Transplant. 2011; 11:896–906.

4. Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients: a systematic review. Transplantation. 2012; 94:775–783.

5. Chung BH, Yang CW. Current issues in the treatment of chronic antibody-mediated rejection in kidney transplantation. Hanyang Med Rev. 2014; 34:211–216.

6. Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9:201–209.

7. Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation. 2008; 86:1754–1761.

8. Yang KS, Jeon H, Park Y, Jo IH, Kim JI, Moon IS, et al. Use of bortezomib as anti-humoral therapy in kidney transplantation. J Korean Med Sci. 2014; 29:648–651.

9. De Sousa-Amorim E, Revuelta I, Diekmann F, Cofan F, Lozano M, Cid J, et al. Bortezomib for refractory acute antibody-mediated rejection in kidney transplant recipients: a single-centre case series. Nephrology (Carlton). 2016; 21:700–704.

10. Lee J, Kim BS, Park Y, Lee JG, Lim BJ, Jeong HJ, et al. The effect of bortezomib on antibody-mediated rejection after kidney transplantation. Yonsei Med J. 2015; 56:1638–1642.

11. Lee HM, Jang IA, Lee D, Kang EJ, Choi BS, Park CW, et al. Risk factors in the progression of BK virus-associated nephropathy in renal transplant recipients. Korean J Intern Med. 2015; 30:865–872.

12. Kim YJ, Jeong JC, Koo TY, Kwon HY, Han M, Jeon HJ, et al. Impact of combined acute rejection on BK virus-associated nephropathy in kidney transplantation. J Korean Med Sci. 2013; 28:1711–1715.

13. McGregor SM, Chon WJ, Kim L, Chang A, Meehan SM. Clinical and pathological features of kidney transplant patients with concurrent polyomavirus nephropathy and rejection-associated endarteritis. World J Transplant. 2015; 5:292–299.

14. Nishio-Lucar A, Balogun RA, Sanoff S. Therapeutic apheresis in kidney transplantation: a review of renal transplant immunobiology and current interventions with apheresis medicine. J Clin Apher. 2013; 28:56–63.

15. Jordan S, Cunningham-Rundles C, McEwan R. Utility of intravenous immune globulin in kidney transplantation: efficacy, safety, and cost implications. Am J Transplant. 2003; 3:653–664.

16. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006; 6:459–466.

17. Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and meta-analysis. Transplantation. 2018; 102:557–568.
18. Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016; 100:391–399.

19. Woodle ES, Tremblay S, Driscoll J. Targeting plasma cells with proteasome inhibitors: principles from primates. J Am Soc Nephrol. 2017; 28:1951–1953.

20. Kim MG, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, et al. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology (Carlton). 2013; 18:820–826.

21. Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012; 81:1067–1074.

22. Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective iterative trial of proteasome inhibitorbased desensitization. Am J Transplant. 2015; 15:101–118.

23. Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011; 91:536–541.

24. Gonzalez S, Escobar-Serna DP, Suarez O, Benavides X, Escobar-Serna JF, Lozano E. BK virus nephropathy in kidney transplantation: an approach proposal and update on risk factors, diagnosis, and treatment. Transplant Proc. 2015; 47:1777–1785.

25. Hodur DM, Mandelbrot D. Immunosuppression and BKV Nephropathy. N Engl J Med. 2002; 347:2079–2080.

26. Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, Mihatsch MJ. Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant. 2009; 24:1024–1033.

27. O'Leary JG, Samaniego M, Barrio MC, Potena L, Zeevi A, Djamali A, et al. The influence of immunosuppressive agents on the risk of de novo donor-specific HLA antibody production in solid organ transplant recipients. Transplantation. 2016; 100:39–53.
28. Nickeleit V, Mihatsch MJ. Polyomavirus allograft nephropathy and concurrent acute rejection: a diagnostic and therapeutic challenge. Am J Transplant. 2004; 4:838–839.

29. Wu D, Zhang MC, Chen JS, Li X, Cheng DR, Xie KN, et al. BK virus-associated nephropathy with plasma cell-rich infiltrates treated by bortezomib-based regimen. Exp Clin Transplant. 2015; 13:603–606.
30. Everly MJ. A summary of bortezomib use in transplantation across 29 centers. Clin Transpl. 2009; 23:323–337.
31. Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017; 91:304–314.

32. Blosser CD, Bloom RD. Recurrent glomerular disease after kidney transplantation. Curr Opin Nephrol Hypertens. 2017; 26:501–508.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download