INTRODUCTION
Post-stroke pain (PSP) is a one of the common complications following a stroke. The reported prevalence of PSP varies, depending on several factors such as study design, definition and classification of pain and characteristics of the participants enrolled in the studies. According to a previous survey, PSP was disclosed by 49.3% of chronic post-stroke patients [
123]. Symptoms in the upper extremity of the affected side in post-stroke patients often have more than one etiology.
The causative conditions include central post-stroke pain (CPSP), complex regional pain syndrome type, pain secondary to spasticity, hemiplegic shoulder pain, and peripheral neuropathy. These make accurate diagnosis and appropriate management challenging for clinicians [
12]. The presence of pain is associated with cognitive decline, increased functional dependence [
4], fatigue [
5], depressed mood, and impaired health-related quality of life [
6].
Therefore, precise identification of etiology through careful inquiry and physical examination is required. In this case report, we describe a post-stroke patient who complained of a tingling sensation in the upper extremity on the affected side. The patient's symptoms had worsened despite rehabilitative therapy and several treatment options started from an early stage were not significantly effective. After attributing the origin of her pain to the scalene muscles, we were able to treat her successfully by applying electrical twitch-obtaining intramuscular stimulation (ETOIMS).
CASE REPORT
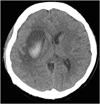
A 41-year-old woman was admitted to our hospital for comprehensive rehabilitation therapy. Four months earlier, she had presented with sudden onset of left-sided weakness. Initial computed tomography (CT) showed an acute hemorrhagic lesion in the right basal ganglia and corona radiata (
Fig. 1). After acute-phase stroke management, she had received rehabilitation therapy for her left-sided weakness.
Fig. 1
CT of the brain demonstrating intracerebral hemorrhage in right basal ganglia and corona radiata.
CT, computed tomography.
At the time of admission, she was mentally alert and scored 28 points in the Korean mini-mental state examination. Muscle strength in the left upper and lower extremities was graded as 2 using the Manual Muscle strength test. The score for spasticity on the Modified Ashworth Scale was grade 1 plus (G1+) in her elbow flexor muscles. She corresponded to stage 3 according to Brunnstrom's recovery stage. She experienced decreased perception of light touch and pinprick sensation on the left side, and deep tendon reflexes were increased on this side. Functionally, she could walk outdoors on a level surface with a cane, and perform activities of daily living under supervision.
At the beginning of the rehabilitation therapy, she noticed a tingling sensation in her left fourth and fifth fingers up to the ulnar side of the forearm. In addition to the physical modalities, she had received pharmacologic treatment with gabapentin 300 mg 3 times daily, pregabalin 75 mg twice daily, and ibuprofen 200 mg 3 times daily until her transfer to our hospital.
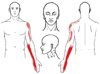
Despite these treatments, her symptoms persisted. At the time of presentation to our hospital, she rated her pain as 3 on a visual analogue scale (VAS) with a range of no pain 0 (no pain) to 10 (worst imaginable pain). While in our hospital, her symptoms gradually worsened with VAS scores reaching 7. Moreover, her symptoms progressed to the upper arm and deltoid muscle area (
Fig. 2).
Fig. 2
Sensory distribution of the area of tingling sensation experienced by the patient.
On physical examination of her left upper extremity, there was no shoulder subluxation and there was full range of motion without pain. There was no evidence of swelling, erythema or decrease in temperature. Atrophied intrinsic muscles of the hand were noted and considered a consequence of the stroke. Palpation of her neck and left upper extremity elicited. Several tender points on the anterior and middle scalene muscles. Compression of these points reproduced her presenting symptoms. Neurologic examinations of the wrist and elbow using provocative tests such as Spurling's test, Phalen's test, and Tinel's sign was normal. The results of blood tests including a complete blood cell count, C-reactive protein, and erythrocyte sedimentation rate were within the normal range. Cervical spine radiography was normal except for loss of the physiologic lordotic curvature. There were no pathologic findings on ultrasonographic examination of the left shoulder.
An electrodiagnostic (EDX) study was performed to rule out cervical radiculopathy and peripheral neuropathies. Nerve conduction studies and needle electromyography were conducted. There were no abnormal findings in any of these tests. As we thought that her increasing symptoms might be attributed to the tendor points on the scalene muscles, we tried to treat the muscles using the technique of ETOIMS.
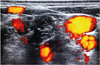
ETOIMS was done with the insertion of a 37-mm monopolar needle electrode using an in-plane technique into the several tender points of the anterior and middle scalene muscles (
Fig. 3). The procedure was performed under ultrasound guidance so as to safely introduce the needle electrodes and avoid damage to the neurovascular bundles (
Figs. 4 and
5).
Fig. 3
The tendor points in left anterior, middle and posterior scalene muscles marked with an “X”.
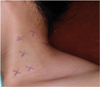
Fig. 4
Imaginary needle path (arrow) to the scalene muscles (asterix) during the ETOIMS using a transverse view.
ETOIMS, electrical twitch-obtaining intramuscular stimulation; SCM, sternocleidomastoid muscle; IJV, internal jugular vein; CCA, common carotid artery.
Fig. 5
The color flow doppler shows the vascular distributions around the neck near the scalene muscles (asterix).
We used the electric source from the nerve stimulator, Stimuplex
® DIG RC (B. Braun Medical Inc., Bethlehem, PA, USA) using the following variables: impulse width of 0.1 ms, frequency 2 Hz, and a current intensity of 1 mA (
Fig. 6). After we noticed twitch response provoked by the needle insertion, ETOIMS was applied for approximately 1 minutes for each tender point.
Fig. 6
Braun Stimuplex® Dig RC (B. Braun Medical Inc., Bethlehem, PA, USA) used as a peripheral nerve stimulator.

Immediately after the ETOIMS, the patient's pain reduced dramatically, with the VAS score decreasing from 7 to 1. Moreover, the pain in the upper arm disappeared. One week after the treatment, the pain remained tolerable, with a VAS score of 2.
DISCUSSION
The mean prevalence of PSP is 29.56%. Although the prevalence of pain subtypes varies according to time course, in general the most frequent type of pain is musculoskeletal including low back and joint pain (24.62%), followed by shoulder pain (9.41%), and CPSP (4.68%) [
7]. However, many patients report more than one pain subtype [
8].
In our patient, the common subtypes of pain in the subacute stage, namely musculoskeletal and shoulder pain, were excluded based on the musculoskeletal ultrasound and X-ray. The symptoms were not characteristic of CPSP and there was no allodynia or dysesthesia to support this diagnosis [
8]. In addition, the possibility of cervical radiculopathy or peripheral neuropathy was low, as there were no specific EDX findings suggestive of these conditions.
As symptoms initially began with rehabilitative treatment and worsened as the amount of the treatment increased, overuse injury was considered as a precipitating factor. The observed tenderness on the scalene muscles, which had not previously been present, seemed to be related to symptoms associated with nerve entrapment.
We considered thoracic outlet syndrome (TOS) as a cause for the symptoms. The clinical presentations of TOS were consistent with her symptoms, and TOS may be caused by compression of neurovascular structures traversing the interscalene triangle formed by the first rib, anterior and middle scalene [
9]. There was a low possibility of scalene myofascial pain syndrome. Because the general pain patterns of referred pain from the scalene muscle trigger points were different from those of our patient [
10]. TOSs are a group of disorders, with one study describing 5 entities as TOSs: arterial TOS, venous TOS, traumatic neurovascular TOS, true neurogenic TOS (TN-TOS), and disputed TOS [
11]. Of these, neurogenic TOS was reported to be more common than vascular TOS, comprising over 90% of all TOS cases [
12]. However, in that series, disputed TOS represented 99% of the neurogenic TOSs [
11]. Also, there is controversy regarding disputed TOS because of its uncertain pathogenesis, unclear clinical presentation, and variable evaluation tools.
Therefore, if there are no obvious abnormal findings during the evaluation process, including the EDX, attention should be paid to the possibility of disputed TOS, as in our case. In disputed TOS, trauma is considered the most common cause. This results in scalene muscle fibrosis, spasm of musculotendinous structures, traction-induced scarring around the brachial plexus, or muscle imbalance, enabling the impingement of neurovascular structures [
1314]. In this case, scalene muscles dysfunctions triggered by repetitive activities causing chronic muscle strain, might explain the symptoms. The symptoms of TOS developed at the beginning of rehabilitation and worsened as treatment intensity increased, with the addition of constraint-induced movement therapy, and robotic devices for upper extremity motor recovery. The compensatory action of proximal muscles instead of the weak distal muscles might have increased the burden on the scalene muscles during treatment.
It is generally recommended that patients with disputed TOS receive conservative treatment initially [
14]. Conservative treatment strategy includes education regarding aggravating factors and postural abnormalities, ergonomic modification, rehabilitative exercises such as stretching programs to promote good posture, and control of body mechanics [
14].
When the patient fails to respond to conservative treatment, non-surgical minimally invasive techniques can be used prior to surgery. These include scalene muscle injection with local anesthetics, steroids, or botulinum toxin A (BTX) [
15]. The intramuscular injection with BTX or steroid might exert its effects in patients with muscle dysfunction by muscle relaxation in the former and anti-inflammatory actions in the latter [
16]. We used the technique of ETOIMS, which induces muscle relaxation, as an alternative to intramuscular injection using anesthetics, steroid or BTX, considering the possible local or systemic side effects of these agents.
Intramuscular stimulation was introduced to diminish muscle tension and provide stimulation to deeply located muscles, especially motor end-plate zones (MEPZ), as both stretching of tight muscles and physical agent modalities using surface electrodes, cannot adequately stretch deep muscle fibers [
16]. Monopolar electromyographic (EMG) needle insertion at MEPZ or neuromuscular junctions leads to electrical activity or micro-twitches. Immediate muscle fiber contraction occurs, followed by relaxation. This may be the basis for the pain relief [
161718].
There are several benefits to the micro-stretch effects of the forceful twitch. Firstly, it reduces mechanical irritations caused by shortened muscles on pain-sensitive neurovascular structures. Secondly, contractile activity maintains the muscle's compliance so that it can work properly. Thirdly, intermittent muscle contraction promotes local blood flow improving tissue oxygenation and removing the accumulation of neurochemicals that may induce pain [
16]. As it uses durable, Teflon-coated monopolar needles, minimizing conduction to surrounding areas, it can facilitate localized stimulation to the target muscles' MEPZ. It also prevents tissues from sticking to the shaft of the needle, reducing the risk of trauma during the movement [
16].
Our patient showed substantial pain relief with ETOIMS in agreement with previous studies demonstrating positive effects of needle ETOIMS in musculoskeletal pain [
1719]. In addition to the conventional methods, we utilized ultrasonogaphy for real-time guidance during the procedure, thereby ensuring safe treatment of the muscles without any damage to nearby neurovascular structures.
This case study has some limitations. Firstly, little is known about long-term or carry-over effects of the treatment as follow-up evaluation was not performed due to loss to follow-up. Secondly, we could not draw any conclusions regarding the superiority of ETOIMS over other techniques. Third, myofascial pain syndrome cannot be completely ruled out although the pain patterns were not compatible with those of our patient.
To the best of our knowledge, this is the first report presenting the effects of ETOIMS in the treatment of a post-stroke patient with disputed TOS. Considering the safety, as well as fewer complications and side effects, we suggest ultrasound-guided ETOIMS as an alternative therapeutic approach for the treatment of patients with disputed TOS due to scalene muscle dysfunction. Based on our case study, further large-scale randomized controlled studies with long-term follow-up are needed to confirm its therapeutic efficacy.











