Abstract
Purpose
Methods
Results
Conclusion
Figures and Tables
Fig. 2
Kaplan-Meier survival curves of N0M1 and N + M1. (A) Overall survivals (OSs) of N0M1 (5-year OS: 49.7% ± 6.2%, n = 76) and N + M1 (25.1% ± 1.7%, n = 759) (P < 0.001, Log-rank test). (B) OSs of N0M1 (5-year OS: 64.9% ± 7.5%) and N + M1 (45.1% ± 2.8%) with curative resection (P = 0.001) (C) OSs of N0M1 (5-year OS: 20.5% ± 8.5%) and N + M1 (vs. 6.4% ± 1.4%) with palliative resection (P = 0.004) (D) Disease free survivals (DFSs) of N0M1 (5-year DFS: 47.4% ± 7.5%) and N + M1 (23.1 ± 2.3) with curative resection (P = 0.001).

Fig. 3
Kaplan-Meier survival curves according to N stages. (A) Overall survivals (OSs) of all (n = 835) patients. The 5-year OS's of N0M1, N1M1, N2aM1, and N2bM1 were 49.7% ± 6.2%, 40.7% ± 3.8%, 29.9% ± 3.2%, and 12.8% ± 2.0% each (P = 0.037 for N0M1 vs. N1M1; P = 0.001 for N1M1 vs. N2aM1; P < 0.001 for N2aM1 vs. N2bM1, Log-rank test). (B) OSs of patients with curative resection. The 5-year OS's of N0M1, N1M1, N2aM1 and N2bM1 were 64.9% ± 7.5%, 57.4% ± 4.8%, 55.4% ± 5.1% and 22.7% ± 4.2% each (P = 0.084 for N0M1 vs. N1M1; P = 0.501 for N1M1 vs. N2aM1; P < 0.001 for N2aM1 vs. N2bM1; P = 0.030 for N0M1 vs. N2aM1). (C) OSs of patients with palliative resection. The 5-year OS's of N0M1, N1M1, N2aM1, and N2bM1 were 20.5% ± 8.5%, 9.1% ± 4.3%, 5.6% ± 2.3%, and 5.9% ± 1.9% each (P = 0.152 for N0M1 vs. N1M1; P = 0.014 for N1M1 vs. N2aM1; P = 0.987 for N2aM1 vs. N2bM1). (D) Disease free survivals (DFSs) of patients with curative resection. The 5-year DFS's of N0M1, N1M1, N2aM1, and N2bM1 were 47.4% ± 7.5%, 31.6% ± 4.4%, 26.2% ± 4.3%, and 12.9% ± 3.2% each (P = 0.081 for N0M1 vs. N1M1; P = 0.140 for N1M1 vs. N2aM1; P = 0.026 for N2aM1 vs. N2bM1; P = 0.005 for N0M1 vs. N2aM1).

Fig. 4
Kaplan-Meier survival curves of patients of N0M1 with curative resection and of stage III. (A) Overall survivals (OSs) of N0M1 (n = 50) with stage IIIA (n = 298), IIIB (n = 2,633), and IIIC (n = 2,521). The 5-year OS's of anyTN0M1, stage IIIa, IIIb, and IIIc were 64.9% ± 7.5%, 87.2% ± 2.2%, 73.2% ± 0.9%, and 47.6% ± 1.1% each (P < 0.001 for anyTN0M1 vs. stage IIIa; P = 0.774 for anyTN0M1 vs. stage IIIb; P = 0.002 for anyTN0M1 vs. stage IIIc, Log-rank test). (B) OSs of N0M1 with stage III according to N stage only. The 5-year OS's of anyTN1M0 (n = 2,932), anyTN2aM0 (n = 1,143), and anyTN2bM0 (n = 1,377) were 74.9% ± 0.9%, 58.3% ± 1.5%, and 38.7% ± 1.4% each (P = 0.587 for anyTN0M1 vs. anyTN1M0; P = 0.063 for anyTN0M1 vs. anyTN2aM0; P < 0.001 for anyTN0M1 vs. anyTN2bM0).

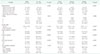
Table 1
Comparison of the clinical characteristics between N0M1 and N + M1 and among subgroups of N + M1

Values are presented as number (%) or mean ± standard deviation.
BMI, body mass index; ASA PS, American Society of Anesthesiologists physical status.
a)Statistical significance between N0M1 and N+M1. b)Statistical significance among N1M1, N2aM1 and N2bM1. c)Synchronous tumors only; Metachronous tumors were not counted. d)In these patients, there were no evidences of peritoneal carcinomatosis except ovary metastasis. e)Tumor size is larger in N2bM1 patients than in N1M1 (Padj = 0.004) and in N2aM1 (Padj = 0.048). f)Padj = 0.006 between N1M1 and N2aM1. Padj = 0.033 between N2aM1 and N2bM1.
Table 2
Comparison of the pathological characteristics between N0M1 and N + M1 and among subgroups of N + M1
Values are presented as number (%) or mean ± standard deviation.
W/D, well differentiated; M/D, moderately differentiated; P/D, poorly differentiated.
a)Statistical significance between N0M1 and N + M1. b)Statistical significance among N1M1, N2aM1 and N2bM1. c)Less lymph nodes were harvested in N2a group than in N1 (Padj = 0.024) and N2b (Padj < 0.001). d)N2bM1 patients had more venous invasions than N1M1 (Padj < 0.001) and N2aM1 (Padj < 0.001).
Table 3
Recurrence rates and first recurrence sites in patients with curative resection

Values are presented as number (%).
a)Statistical significance between N0M1 and N + M1. b)Statistical significance among N1M1, N2aM1 and N2bM1. c)Chi-square test was done with exclusion of patients with unavailable recurrence data. d)Fisher exact test. e)N2bM1 patients had more recurrence than N1M1 (Padj < 0.001).
Table 4
Postoperative chemotherapy regimensa)

Values are presented as number (%).
a)Chi-square test for chemotherapy or not between N0M1 and N + M1 did not reveal significant difference (P = 0.147). However, chi square test for chemotherapy or not among N stages (N0–N2b) showed significant difference (P = 0.01) and post hoc test revealed less patients underwent chemotherapy in N2bM1 (282/343 = 82.2%) than N1M1 (188/203 = 92.6%) (Padj = 0.024). However, when the patients were separated to the curative resection or palliative resection, there were no statistical differences in comparisons of chemotherapy or not between N0M1 and N + M1 or among N stages (N0–N2b), each.
Fisher exact test for chemotherapy regimens between N0 and N+ or among N stages (N0–N2b) revealed no statistically significant differences (P > 0.05). When the patients were separated to curative or palliative resection, there were not statistical differences either.
Table 5
Clinicopathological factors contributing to overall survivals of patients with curative or palliative resection applied by Cox proportional hazard model with enter method

HR, hazard ratio; CI, confidence interval; BMI, body mass index; ASA PS, American Society of Anesthesiologists physical status; W/D, well differentiated; M/D, moderately differentiated; P/D, poorly differentiated.
a)Factors of P < 0.1 in the univariable analysis are included in the multivariable analyses. b)If multivariable analysis was calculated with Log10CEA, the hazard ratio is 1.237 (1.089–1.405). c)Patients with adenocarcinoma well differentiated (0.301 [0.126–0.722]), moderately differentiated (0.287 [0.138–0.597]) and poorly differentiated (0.283 [0.128–0.623]) had low hazard ratio than patients with mucionus carcinoma. d)Patients of N1M1 have low hazard ratio than patients of N2aM1 (0.629 [0.448–0.883]).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download