Abstract
Purpose
This study evaluated midterm changes in body composition after open distal gastrectomy for early gastric cancer.
Methods
Data from 138 gastric cancer patients who underwent open distal gastrectomy at Kyungpook National University Chilgok Hospital between January 2011 and December 2012 were collected and reviewed. Patients with pathological stage I gastric cancer and with no comorbidities at diagnosis were enrolled. Body composition data from segmental multifrequency bioelectrical impedance analysis were obtained preoperatively and at 1, 2, and 3 years after surgery.
Results
The mean body weight losses at 1 and 3 years after surgery were 6.1 kg and 5.8 kg, respectively (P < 0.001). The protein mass, skeletal muscle mass, and fat-free mass decreased continuously until 3 years after surgery (0.5 kg, 1.6 kg, and 2.4 kg, respectively; P < 0.001). The average body fat mass and visceral fat area loss at 1 year after surgery were 4.7 kg and 20.5 cm2, respectively (P < 0.001). After 1 postoperative year, the body fat mass and visceral fat areas increased continuously, up to 12.2 kg and 74.2 cm2 at 3 years after surgery, respectively (+1.4 kg and +1.2 cm2, respectively).
Body composition changes are common phenomena after gastrectomy. Especially, weight loss is a remarkable problem after radical surgery for gastric cancer. The main mechanism of weight loss is impaired food intake and malabsorption [1]. Several studies have reported that weight loss occurs predominantly during the first 3 months after gastrectomy, and the weight is not generally regained during the first postoperative years [23]. Further, gastric surgery is associated with decreases in the metabolically active body mass, related to the considerable changes in nutritional status. Alteration of nutritional status after gastrectomy is considered an unavoidable complication, and the postoperative nutritional condition is one of the pivotal factors affecting the prognosis and quality of life in gastrectomized patients.
Assessment of body composition serves an important role in nutritional evaluation to define prognosis in numerous diseases [4]. It is especially important to evaluate postoperative body composition changes after gastrointestinal surgery, and the prognosis is intimately related to each individual patient's nutritional status. There are several methods for analyzing body composition, including body mass index (BMI), bioelectrical impedance analysis using wrist-ankle resistance, and dual energy X-ray absorptiometry [5]. Among these, bioelectrical impedance analysis appears to provide a non-invasive, safe, rapid, and accurate method for assessing body composition [4]. The body composition model can be divided into the 2-component and 4-component models [6]. The 4-component model, in which the body is divided into fat mass, total body water, protein, and minerals, is more practical in clinical assessment than the 2-component model, in which the body is divided into fat mass and fat-free mass (FFM). Previous studies have reported the usefulness of bioelectrical impedance analysis in assessing the body composition and body composition changes in a wide range of physiological and clinical conditions [789].
Although several studies have reported body composition changes after gastrectomy using bioelectrical impedance analysis within one year postoperatively [10111213], there are currently few data regarding the changes in body composition after more than 1-year postgastrectomy. In addition, the standard procedure for early gastric cancer is a minimally invasive surgery at present, however, we first tried to identify changes in body composition in open surgery. Therefore, the aim of this study was to evaluate the chronological changes in body composition after open distal gastrectomy during the first three postoperative years.
We enrolled 337 patients who underwent open distal gastrectomy for primary gastric cancer between January 2011 and December 2012 at Kyungpook National University Chilgok Hospital. Among them, 284 patients were diagnosed with pathological stage I gastric cancer. We excluded 114 patients with comorbidities, such as cardiovascular disease, chronic liver disease, chronic renal disease, diabetes mellitus, chronic respiratory disease, and cerebrovascular disease. Among the remaining patients with stage I gastric cancer, 138 patients who completed the entire series of body composition assessments preoperatively and annually for 3 years postoperatively were analyzed. The patients were divided into subgroups by sex (male vs. female) and BMI (<23 kg/m2 vs. ≥23 kg/m2). Informed consent was obtained from all participants, and the study design was approved by the Institutional Review Board of Kyungpook National University Chilgok Hospital (approval number: 201606020). The study was conducted in accordance with the Declaration of Helsinki.
Reconstruction was performed by Billroth I gastroduodenostomy using a circular stapler. Total omentectomy and D2 lymph node dissection were performed in all patients.
Body composition was assessed by segmental multifrequency bioelectrical impedance analysis using InBody 720 (Biospace, Seoul, Korea) preoperatively and annually up to 3 years postoperatively. According to the manual provided by the manufacturer, the patients were examined in the morning, prior to having breakfast and exercising, if possible. The patients stood in an upright position when stepping on the foot electrodes, gripping the hand electrodes loosely. In this position, the eight tactile electrodes were placed in contact with the thumb and palm of each hand and the front and rear soles of each foot. An electrical scale was used to measure the height and weight of each patient. Bioelectrical impedance analysis measures the opposition of body tissues to the flow of a small alternating current. Impedance is a function of 2 components: the vector sum of resistance and the reactance. The measured resistance is approximately equivalent to that of muscle tissue. As total body water is distributed mainly in the muscle, FFM can be measured by calculating the total body water. When calculating FFM, body fat mass (BFM) can be calculated by subtracting the FFM from the body weight. Thus, the impedance method measures the body composition through the resistance of the body [5]. The BMI was calculated as body weight/height2 (kg/m2), and the degree of obesity was calculated as body weight/ideal body weight (%).
Differences among time intervals were determined with repeated-measures 1-way analysis of variance (RM-ANOVA). A P-value of <0.05 was considered statistically significant. Differences between the 2 groups were analyzed with RM-ANOVA with the post hoc tests and Student t-test. Bonferroni's method was used for the post hoc tests and to correct the level of significance. The significant P-value for these comparisons was set to equal to 0.013 (0.05/4) when compared between the two groups for the four time points. All statistical analyses were conducted using SPSS software (version 22; SPSS, Chicago, IL, USA).
Table 1 presents the demographic characteristics of the patients. The mean age was 56.9 ± 11.5 years. There were 47 female and 91 male patients. The BMI < 23 and ≥ 23 kg/m2 groups comprised 71 and 67 patients, respectively. The mean hospital stay was 10.3 ± 5.5 days. Morbidity occurred in 8 patients. Intra-abdominal fluid collection was seen in 4 patients and all were treated with percutaneous drainage. Ileus occurred in 2 patients, both of whom were treated with nasogastric decompression. Anastomosis leakage occurred in 2 patients; both patients underwent conversion to Billroth II anastomosis. After reoperation, both patients were discharged without complications. No patients showed evidence of recurrence at the final follow-up.
Fig. 1 shows the changes in the mean body weight, BMI, BFM, and visceral fat areas (VFAs). The average body weight losses at 1 and 3 years after surgery were 6.1 kg and 5.8 kg, and the relative body weights at 1 and 3 years, as compared to preoperatively, were 90.3% and 90.8%, respectively, with statistical significances (P < 0.001). The changes in BMI were similar to the changes in body weight. The average BFM and VFA losses at 1 year postoperatively were 4.7 kg and 20.5 cm2, and the relative BFM and VFAs at 1 year compared to those preoperatively were 69.7% and 78.1%, respectively (P < 0.001). Between 1 and 3 years after surgery, the BFM and VFAs increased continuously up to 12.2 kg and 74.2 cm2, and the relative BFM and VFAs at 3 years compared to preoperatively were 78.7% and 79.4%, respectively.
Fig. 2 shows the changes in the mean protein mass (PM), skeletal muscle mass (SMM), FFM, and waist-hip ratio. The PM, SMM, and FFM continuously decreased during the first 3 years after surgery. The average PM, SMM, and FFM losses at 3 years after surgery were 0.5 kg, 1.6 kg, and 2.4 kg, and the relative PM, SMM, and FFM at 3 years compared to preoperatively were 94.6%, 93.9%, and 94.9%, respectively (P < 0.001). The waist-hip ratio was decreased by 3.4% during the first year after surgery and was maintained in a reduced state throughout the followup period.
Comparing the changes in body composition by sex, all parameters showed no significant differences between the male and female groups, except in waist-hip ratio (Figs. 3, 4). The waist-hip ratio was significantly more decreased in the female group than in the male group at 2 years after surgery (95.0% vs. 97.0%, P = 0.002).
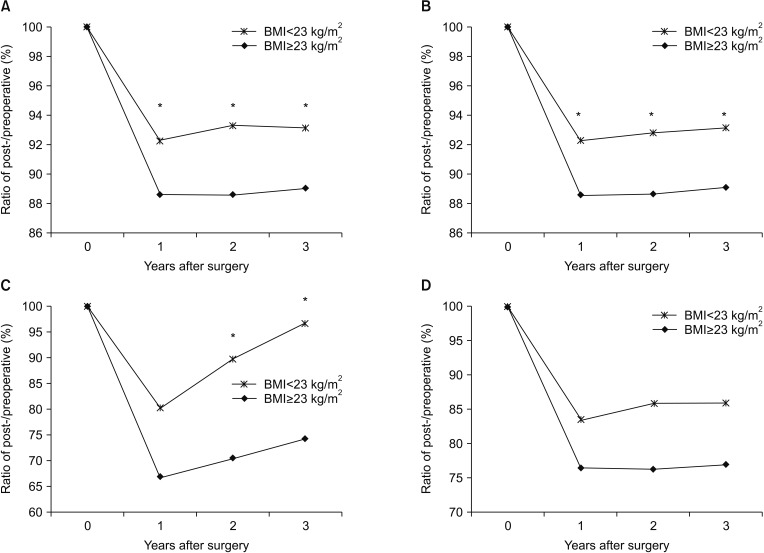
Comparing the changes in body composition by a BMI cutoff of 23 kg/m2, no parameters showed a significant difference between the BMI < 23 and ≥ 23 kg/m2 groups, except body weight and BFM. The body weight reduction was significantly larger in the BMI ≥ 23 kg/m2 group than in the BMI < 23 kg/m2 group at all time points after surgery. The BFM was decreased during the first year after surgery, without a significant difference between the 2 groups. Thereafter, the BFM had a tendency to recover more significantly in the BMI < 23 kg/m2 group than in the BMI ≥ 23 kg/m2 group (Figs. 5, 6).
This study investigated the changes in body composition of patients undergoing open distal gastrectomy during the first 3 years after surgery. Several studies have reported that the body weight, PM, and BFM after gastrectomy decreased during the immediate postoperative period and continuously decreased until 6 months after surgery. Moreover, it is well-known that the main contributor to body weight loss is BFM loss rather than PM loss in this period. Six months after gastrectomy, the body weight was maintained or slightly regained in the previous reports [10111214]. Beyond previous studies, this study was conducted to assess all body composition changes up to 3 years after gastrectomy. In this study, the body weight decreased the most during the first year after surgery and was subsequently maintained at that level during the next 2 years postoperatively. The BFM was most decreased during the first postoperative year, after which it continuously recovered. The changes in PM, SMM, and FFM showed similar patterns after surgery and decreased continuously through the first 3 postoperative years; however, the rate of decrease diminished 1 year after surgery. As shown in this study, changes in body composition items might be classified into 2 categories: (1) the body composition items that are significantly decreased during the first postoperative year and are maintained at this level or are slightly increased during the next 2 years postoperatively, such as body weight, BMI, waist-hip ratio, BFM, and VFAs and (2) the body composition items that are significantly decreased during the first postoperative year and continuously decrease through the first 3 years postoperatively, such as PM, SMM, and FFM.
Changes in body weight, BMI, BFM, and VFAs are related to the balance and control of our bodies. The regulation of body weight is affected by the balance and interaction between food intake and energy expenditure. The compensation mechanism of changing energy expenditure works to prevent persistent weight changes. Reducing energy expenditure after gastrectomy not only serves to impede continuous weight loss but also to decrease the energy demand in our body. Further, it regulates the energy expenditure for the lower food intake after gastrectomy [1516]. Oxidation of fat is involved in this process to supplement depletion of energy during the first year after gastrectomy. One year after surgery, stabilization of fat oxidation occurs to maintain balanced body composition [16]. Likewise, in the results of this study, the BFM and VFAs decreased significantly during the first postoperative year and slightly increased after then due to stabilization of fat oxidation. This phenomenon might influence the weight of the patients, which significantly decreased during the first postoperative year and was maintained at that level throughout the next 2 years.
Despite the BFM recovery observed after 1 postoperative year, the body weight is not generally regained, owing to the continuous decreases in PM and SMM. After surgery, proteolysis is accelerated to produce amino acids for the support of gluconeogenesis. Consumption of endogenous protein leads to decreased PM after gastrectomy. The continuous decrease in PM after gastrectomy is also affected by protein maldigestion, which is associated with low gastric enzyme levels and intestinal bacterial overgrowth [171819]. The changes in SMM are related to muscle atrophy, which may be predisposed by the altered ratio of serum concentrations of essential and branched-chain amino acids after gastrectomy [20]. In addition, the muscle mass after gastrectomy may be affected by the patient's physical activity or postoperative in-bed immobility [12]. The changes in skeletal muscle play a role in mediating the alterations in energy expenditure [15]. Despite of the decreased PM and SMM after gastrectomy, the body composition is stabilized by reducing the energy expenditure mechanism and gradually increasing dietary intake during the first year after surgery. As a result, severe protein-calorie malnutrition rarely occurs after gastrectomy. However, improvements in PM and SMM are hard to attain without exogenous factors such as intake of protein-containing food or muscle-strengthening exercises. Regular education programs on diet, as well as an accelerated rehabilitation program, are mandatory after gastrectomy.
It is well-known that males have more VFAs than females, whereas females have more BFM and less FFM than males [2122]. Although the body composition differs between the sexes, this study revealed that almost all parameters showed no significant differences between males and females. There were no significant differences in BFM and VFAs between the 2 groups. However, the waist-hip ratio, which represents the degree of visceral fat, revealed a significant difference. We supposed that the relation between physical activity energy expenditure and percentage of body fat, as well as the difference in compensation mechanism of insufficient energy, between males and females might have affected the results.
The criteria of BMI 23 kg/m2 as the cutoff was determined based on a World Health Organization expert panel report [23]. In this study, the BMI < 23 kg/m2 group showed less body weight change and more recovery in BFM during the follow-up period. Our results showed only the differences in continuous body composition changes between the 2 groups during 3 postoperative years, rather than comparing the outcomes between groups. Through these 2 subgroup analysis, changes in body composition may vary depending on the characteristics of the patient such as sex and BMI even if the same operation was performed. However, it is difficult to obtain sufficient evidence from this study to determine which tailored medical intervention to perform for each patient. Based on our results, we are planning to study about postoperative body composition change according to nutrition and exercise support after gastrectomy.
This study might suggest a significant clinical parameter in patients who underwent gastrectomy. Postoperative body composition change is not a static change due to operation but rather an ongoing change with a significant gap compared with the preoperative body composition status. To prevent ongoing change in body composition after gastrectomy, previous studies suggested that nutritional improvement by dietary education and counselling is an effective intervention for achieving better outcome and quality care in patients undergoing gastrectomy [24]. Furthermore, intervention with dietary advice and/or oral nutritional supplements could be tried to improve the nutritional state in malnourished patients with cancer [25]. In the management of weight loss in gastrectomy patients, dietary advice and supplements, such as consuming a balanced diet, resolving any fears of eating, keeping a dietary diary, and high protein- and energy-containing supplements, are beneficial to weight gain. However, the optimal period of nutritional intervention is unknown. On the basis of the results of this study, intensive nutritional intervention during the first year after gastrectomy is mandatory to prevent weight loss, and particular attention to improve the PM and SMM would be advisable.
The current study had several potential limitations due to its retrospective nature. First, only patients who had been diagnosed with stage I gastric cancer and without underlying diseases were included. Body composition might be affected by various conditions, including cardiovascular disease, hepatic disease, and tumor burden. However, determining the changes in nutrition physiology after open distal gastrectomy might provide baseline data that can be adjusted in patients with underlying diseases or diagnosed with advanced gastric cancer after gastrectomy. Second, as this study is a single institution study based on an Asian population, the patient characteristics and range of BMI included may not be representative of other, especially Western, populations. Thus, the results from our study need to be validated in Western countries.
The body weight was significantly decreased during the first year after open distal gastrectomy, and the PM and SMM decreased continuously during the 3 postoperative years in our study cohort. More intense nutritional and exercise programs may be important after gastric cancer surgery, especially during the first postoperative year.
References
1. Armbrecht U, Lundell L, Lindstedt G, Stockbruegger RW. Causes of malabsorption after total gastrectomy with Roux-en-Y reconstruction. Acta Chir Scand. 1988; 154:37–41. PMID: 3354282.
2. Adams JF. The clinical and metabolic consequences of total gastrectomy. I. Morbidity, weight, and nutrition. Scand J Gastroenterol. 1967; 2:137–149. PMID: 20184481.
3. Katsube T, Konnno S, Murayama M, Kuhara K, Sagawa M, Yoshimatsu K, et al. Changes of nutritional status after distal gastrectomy in patients with gastric cancer. Hepatogastroenterology. 2008; 55:1864–1867. PMID: 19102410.
4. Lorenzo AD, Andreoli A. Segmental bioelectrical impedance analysis. Curr Opin Clin Nutr Metab Care. 2003; 6:551–555. PMID: 12913672.

5. Jaffrin MY, Morel H. Body fluid volumes measurements by impedance: a review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Med Eng Phys. 2008; 30:1257–1269. PMID: 18676172.

6. Heymsfield SB, Lichtman S, Baumgartner RN, Wang J, Kamen Y, Aliprantis A, et al. Body composition of humans: comparison of two improved four- compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990; 52:52–58. PMID: 2360552.
7. Brodie D, Moscrip V, Hutcheon R. Body composition measurement: a review of hydrodensitometry, anthropometry, and impedance methods. Nutrition. 1998; 14:296–310. PMID: 9583375.

8. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am J Clin Nutr. 1996; 64(3 Suppl):524S–532S. PMID: 8780375.
9. De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol (1985). 1997; 82:1542–1558. PMID: 9134904.

10. Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005; 9:313–319. PMID: 15749590.

11. Liedman B, Andersson H, Bosaeus I, Hugosson I, Lundell L. Changes in body composition after gastrectomy: results of a controlled, prospective clinical trial. World J Surg. 1997; 21:416–420. PMID: 9143575.

12. Abdiev S, Kodera Y, Fujiwara M, Koike M, Nakayama G, Ohashi N, et al. Nutritional recovery after open and laparoscopic gastrectomies. Gastric Cancer. 2011; 14:144–149. PMID: 21327442.

13. Park KB, Kwon OK, Yu W, Jang BC. Body composition changes after totally laparoscopic distal gastrectomy with delta-shaped anastomosis: a omparison with conventional Billroth I anastomosis. Surg Endosc. 2016; 30:4286–4293. PMID: 26823058.
14. Hwang SE, Kim CY, Yang DH. Changes in body composition after a radical gastrectomy for a gastric adenocarcinoma using bioelectrical impedance analysis during the first year following surgery. J Korean Gastric Cancer Assoc. 2007; 7:228–236.

15. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995; 332:621–628. PMID: 7632212.

16. Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000; 85:1087–1094. PMID: 10720044.
17. Iivonen MK, Ahola TO, Matikainen MJ. Bacterial overgrowth, intestinal transit, and nutrition after total gastrectomy. Comparison of a jejunal pouch with Rouxen-Y reconstruction in a prospective random study. Scand J Gastroenterol. 1998; 33:63–70. PMID: 9489910.
18. Murawa D, Murawa P, Oszkinis G, Biczysko W. Long-term consequences of total gastrectomy: quality of life, nutritional status, bacterial overgrowth and adaptive changes in esophagojejunostomic mucosa. Tumori. 2006; 92:26–33. PMID: 16683381.

19. Bragelmann R, Armbrecht U, Rosemeyer D, Schneider B, Zilly W, Stockbrugger RW. Nutrient malassimilation following total gastrectomy. Scand J Gastroenterol Suppl. 1996; 218:26–33. PMID: 8865447.
20. Saito A, Noguchi Y, Yoshikawa T, Doi C, Fukuzawa K, Matsumoto A, et al. Gastrectomized patients are in a state of chronic protein malnutrition analyses of 23 amino acids. Hepatogastroenterology. 2001; 48:585–589. PMID: 11379360.
21. Tershakovec AM, Kuppler KM, Zemel B, Stallings VA. Age, sex, ethnicity, body composition, and resting energy expenditure of obese African American and white children and adolescents. Am J Clin Nutr. 2002; 75:867–871. PMID: 11976160.

22. Paul DR, Novotny JA, Rumpler WV. Effects of the interaction of sex and food intake on the relation between energy expenditure and body composition. Am J Clin Nutr. 2004; 79:385–389. PMID: 14985211.

23. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004; 363:157–163. PMID: 14726171.
24. Kim H, Suh EE, Lee HJ, Yang HK. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: a randomized controlled trial. Cancer Nurs. 2014; 37:E10–E20. PMID: 23632471.
25. Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012; 104:371–385. PMID: 22345712.

Fig. 1
Comparison of changes in body composition of patients undergoing open distal gastrectomy: (A) body weight, (B) body mass index, (C) body fat mass, and (D) visceral fat areas.
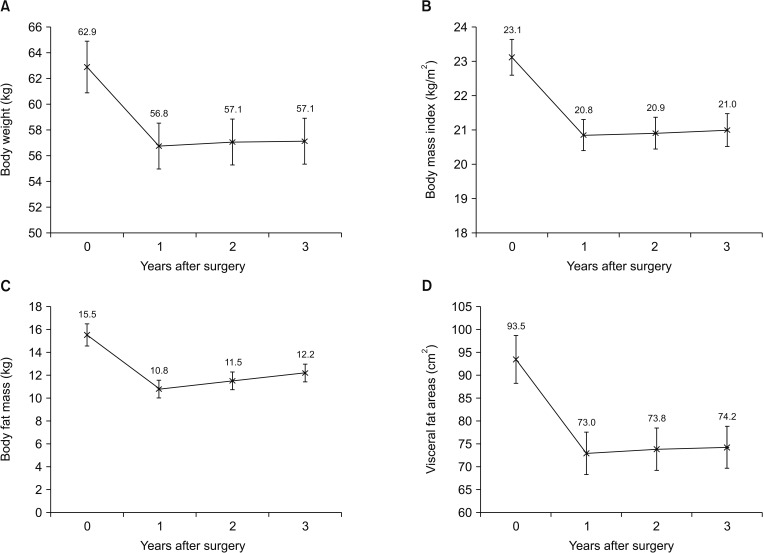
Fig. 2
Comparison of changes in body composition of patients undergoing open distal gastrectomy: (A) protein mass, (B) skeletal muscle mass, (C) fat-free mass, and (D) waist-hip ratio.
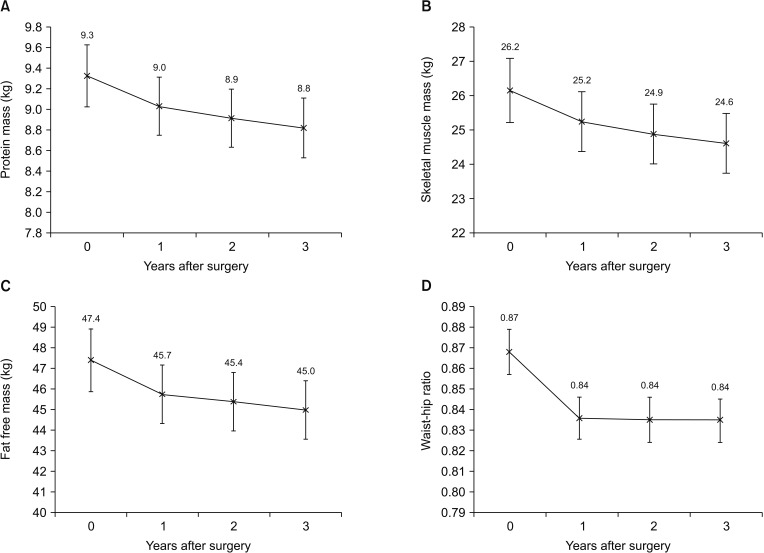
Fig. 3
Comparison of the rates of changes in body composition of the patients according to sex: (A) body weight, (B) body mass index, (C) body fat mass, and (D) visceral fat areas.
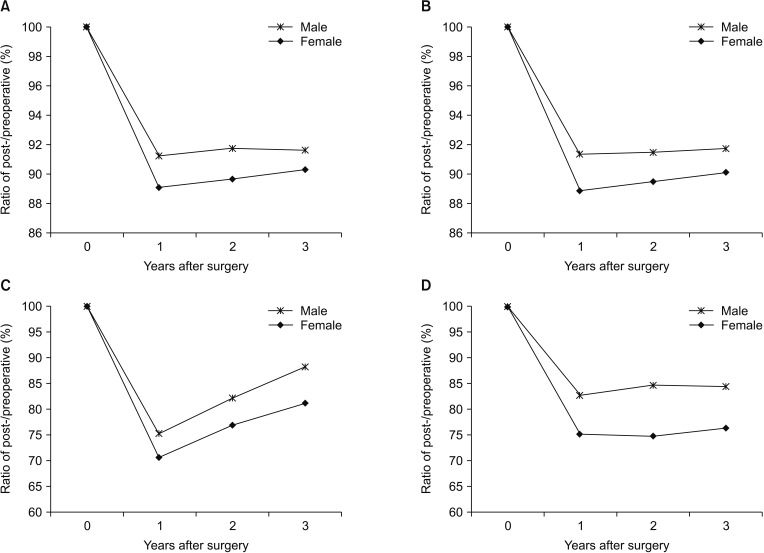
Fig. 4
Comparison of the rates of changes in body composition of the patients according to sex: (A) protein mass, (B) skeletal muscle mass, (C) fat-free mass, and (D) waist-hip ratio. *P < 0.05.
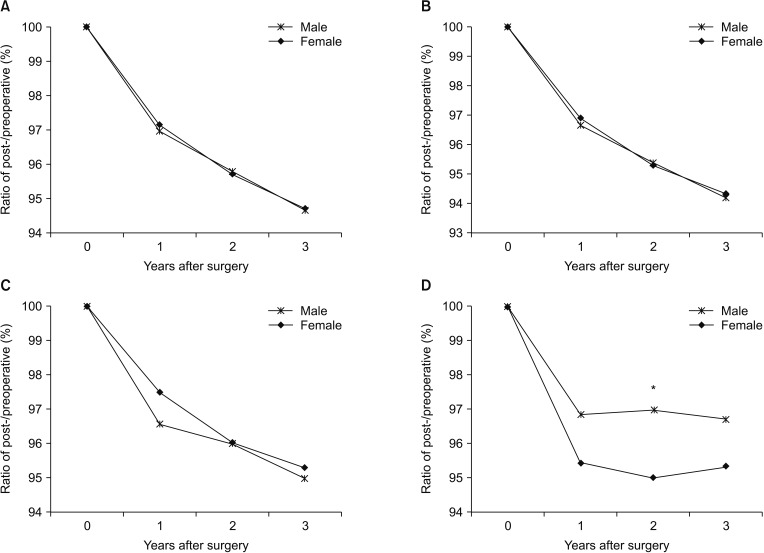
Fig. 5
Comparison of the rates of changes in body composition of the patients according to body mass index: (A) body weight, (B) body mass index (BMI), (C) body fat mass, and (D) visceral fat areas. *P < 0.05.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download